
olivertwist - 4-2-2008 at 10:13
hi all. i'm having a little trouble with an o chem problem for school. we have to draw all the resonance structures for p-dimethylaminophenol and
p-nitrophenol. i think i'm on the right track, but do i start moving electrons from the OH group toward the N or the other way? any help would be
great.
Ramiel - 4-2-2008 at 13:05
The way I looked at it wasn't as 'electrons moving' but rather, electron density.
There are a few special cases where electrons or electron pairs really do move around a molecule, but in most cases of resonance, I
<i>think</i> that they don't move.
Resonance drawings are not 'real', but more a thought exercise. It shows you the overall electron density as a combination of the acceptable resonance
structures. Think of it as a method for overcoming the weakness of simple lewis structure drawings in organic (oh, and inorganic) chemistry.
I remember being reminded that 'electron pushing' a'la reaction mechanisms are not strictly true, but still are useful in predicting a mechanism.
So in your case, both the alcohol and amine/nitro groups are electron donating. You'll get a combination of benzenes with a delocalised negative
charge from the amine/nitro and alcohol.
p.s. Yes, I was both rewarded and reviled for my creative use of dotted lines, curved 'multi-bonds' and some shading in org.chem.
olivertwist - 4-2-2008 at 14:17
thanks for your help. i know that the structures aren't "real" but i still have to be able to draw them for the test. i also understand that they will
delocalise but for the purpose of the test, do you start at the -OH end or the N?
sparkgap - 4-2-2008 at 14:50
All you have to remember is that for the purposes of "electron pushing" you have to 1) identify which groups are electron rich and electron poor; and
2) push electrons in the direction of "electron rich to electron poor."
So, for the example of p-nitrophenol, you start with those lone pairs in the hydroxy group...
sparky (~_~)
AndersHoveland - 12-11-2011 at 10:35
This should give some idea about how resonance works
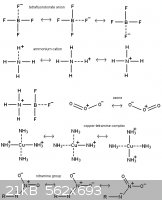
[Edited on 12-11-2011 by AndersHoveland]
