amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
Some novice questions
1-If I want to carry out a chemical reaction at a certain low temp (say -30 or -40) ......how to get such a temperature using cardice or liquid
nitrogen? (i.e I want a method to control the temp using ice solvent mixture or any feasible cheap method)
2-How to maintain such temperature if I have small dewar (about 2 liters) and at the same time I need to stir the reaction mixture?
3-I need to carry out a reaction under pressure ( about 4 hours at 125 degree Celsius ) and I need a sealed tube to do so or autoclave ...........Is
there any DIY or easier way to get such pressure or someway to get a cheap sealed tube for that can endure such mild conditions?
[Edited on 14-3-2008 by amrhamed2]
amr h mahmoud
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
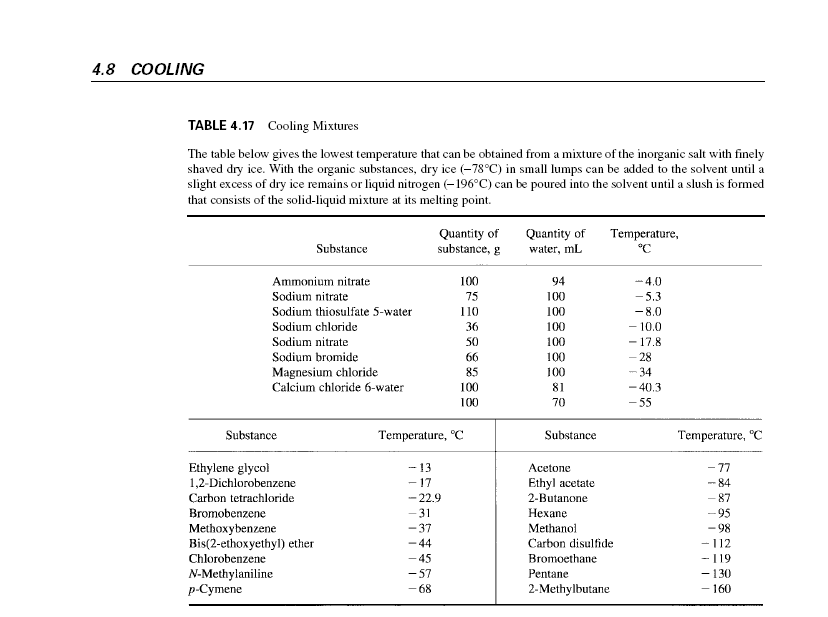
References such as Lange's Handbook and many lab techniques textbooks have tables of liquids and mixtures that can be frozen with liquid nitrogen or
solid CO2, then allowed to melt giving nearly constant temperature at that melting point. They also list ice-salt mixtures to achieve specifics
temperature ranges, -40 is getting too cold for most of these. The high tech way is to use a low temperature chiller to circulate a coolant at the
desired temperature.
You just stir it. It's common to close the tops of dewars and chilled vessels with loose fitting plugs of styrofoam or cork, just leave a hole to
thread the stirring shift through.
Again lab technique books, especially those from the 1950s and earlier, describe the use of sealed tubes. Ordinary waterpipe can withstand a moderate
amount of pressure if the temperature isn't too high, the smaller the diameter the more it can take. Of course open containers of materials that
attack iron and steel don't do well inside such a pipe.
For only 2 PSI, less than a quarter bar, many types of heavy walled labware will do. The manufactures often have information on the maximum negative
and positive pressure that should be applied to given products. If all else fails, heavy walled borosilicate tubing can be used, that nearly always
has maximum pressure specifications given.
this PDF shows some examples of pressure labware
http://www.aceglass.com/featured/pressure_tubes/pressuretube...
and a table of maximum internal pressure for typical borosilicate tubing in various diameter and wall thicknesses
http://www.udel.edu/chem/GlassShop/BorosilicateMAIPTable.htm
[Edited on 14-3-2008 by not_important]
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
Thanks very much
Here is a link to Lang Handbook
h##p://rapidshare.com/files/99462536/lange_s.handbook.of.chemistry.rar
amr h mahmoud
|
|
|