joniaguis
Harmless

Posts: 15
Registered: 2-5-2009
Member Is Offline
Mood: No Mood
|
|
Nicotine
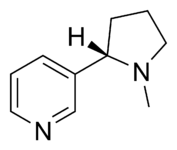
Nicotine has pKa values of 6.16 and 10.96. Assign these to the appropriate atoms in nicotine and suggest the structure of nicotine that predominates
in plasma (pKa 7.4)
Any help pls?
|
|
|
chemoleo
|
Thread Moved
6-5-2009 at 15:23 |
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Well you know the structure of Nicotine?
Nicotine has two tert-amine nitrogens.
Nitrogens in an aromatic system have a lower pKa than those of aliphatic systems.
Pyridine pKa is 5.21 based on this I would assign the pyridinyl N of nicotine 6.16 and the pyrrolidinyl N 10.96
pH of blood plasma is ca 7.4 so the pyridinyl N will be uncharged and the pyrrolidinyl N will be cationic.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Consider the conjugate acid of both nitrogens individually. Which is more stable? (consider hybridisation/delocalisation/hyperconjugation/inductive
effects) This more stable acid is the weaker acid, thus implying it has a higher pKa.
panziandi is correct in saying that the pyridinyl N of nicotine has the pKa of 6.16 and the pyrollidinyl N has the pKa of 10.96. However his reasoning
is not great, (although along the right lines) and in an exam would get you little marks.
The pyridinyl N is sp2 hybridised, the lone pair therefore lies in the plane of the pyrindine ring. The pyrrolidinyl N is sp3 hybridised and so the
lone pair lies in the vacant tetrahedral position. Both lone pairs are aprroximately equally hindered so why is one much stronger (10000x) than the
other?? The answer is hybridisation. The pyrolidinyl N has its lone pair in an sp3 orbital, but the pyridinyl N has the lone pair in an sp2 orbital.
The sp2 orbital has more s character (in terms of the orbitals that comprise it, one s and 2 p, compared to one s and 3 p for an sp3 hybrid).
s orbitals are deeply penetrating and so the electrons are held much closer to the N nucleus in an sp2 hybrid than in an sp3 hybrid, reducing their
ability to act as a base and pick up a proton, thus pyridine is a weaker base than pyrolidine and hence a stronger (conjugate) acid than pyrollidine
(i.e. pyridine has a lower pKa than pyrollidine)
|
|
|
joniaguis
Harmless

Posts: 15
Registered: 2-5-2009
Member Is Offline
Mood: No Mood
|
|
again, thanks dj
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
See http://en.wikipedia.org/wiki/Nicotine ,
which gives a good summary, with references, of its chemical, pharmacological, insecticidal, and toxicological properties. I am wanting to grow my
own tobacco, to extract nicotine from it for use as an insecticide (except on flowering plants in season to which bees may be attracted).
|
|
|
joniaguis
Harmless

Posts: 15
Registered: 2-5-2009
Member Is Offline
Mood: No Mood
|
|
compare the ease of reaction of the oxidation of furan with that of benzene with KMnO4.
Furan is an activated ring but how does oxidation happen? On the beta positions??
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JohnWW  | | I am wanting to grow my own tobacco, to extract nicotine from it for use as an insecticide (except on flowering plants in season to which bees may be
attracted). |
Nicotine is a broad-spectrum insecticide that plays total havoc on insect ecosystems in gardens.
The "California Certified Organic Farmer" (CCOF) program specifically forbids nicotine as a CCOF-permissible insecticide, for example. Grow nicotine
if you care to, but have you considered growing pyrethrin instead?
|
|
|