towards a less hydrophobic synthetic polymer
Synthetic polymers generally do not contain hydroxy groups as such. The exceptions are usually polymers which have been post-treated for
hydrophilicity, such as polyvinyl alcohol derived from hydrolysis of polyvinyl acetate. Alternatively, synthetic derivatives of natural polymers such
as rayon or chitosan can be used. Unfortunately, these polymers generally have inferior durability and chemical stability when compared with natural
fibers or most synthetic polymers.
This is because polymerization generally happens in one of two ways, which usually do not tolerate hydroxy groups:
- addition polymerization, such as ROMP and alkene polymerization, which generally depends on radical, carbocation or carbene intermediates, which are
poisoned by hydroxy groups
- condensation polymerization, such as the production of nylon, PETE or kevlar, which usually uses a condensing agent like P2O5 which will react with
hydroxy groups
The challenge is to find a polymerization reaction which is not affected by the presence of hydroxy groups. The most attractive examples are
cyclocondensation reactions, but many of these lead to unstable materials: 3+2 cycloadditions based on azides or nitrones give triazoles and
isoxazolines respectively, which are relatively unstable or even potentially explosive. The Diels-Alder reactions usually require forcing conditions
and leave behind a reactive alkene. Many others require relatively unstable precursors like haloketones, aldehydes and beta-ketoesters. There is one
exception:
http://en.wikipedia.org/wiki/Paal-Knorr_pyrrole_synthesis
The reaction of 1,4-diketones with amines forms pyrroles under mild conditions with high selectivity. The reaction eliminates water but tends to be
favorable even in aqueous solution. So the natural choice of precursors is a bis(1,4-diketone) and a diamine. The obvious choice for the latter is
p-phenylenediamine.
The choice of a bis-(1,4-diketone) containing hydroxy groups is less obvious. After some thought I realized that reacting a (bis)diene with
1,2-diacetylethylene would give the required ketones and dihydroxylation of the byproduct alkenes with e.g. KMnO4 or OsO4 would give the hydroxy
groups.
There are some possibilites for the bis(diene), of which cyclooctatetraene is certainly the easiest to synthesize by tetramerization of acetylene.
However, this does not undergo two Diels-Alder reactions since the reaction begins with isomerization to a bicyclo[4.2.0]octa-1,3,7-triene. More
realistic possibilities are 1,2-bis(cyclopentadienyl)ethane and 1,2-bis(2-butadienyl)ethane, formed as below:
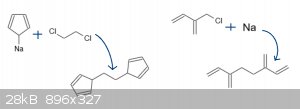
(alkylation of cyclopentadienide / Wurtz coupling of 2Me-chloroisoprene)
After Diels-Alder reaction with two equivalents of 1,2-diacetylethylene aka 3-hexen-2,5-dione, and hydroxylation, the monomer based on the second
compound looks like this (the other one is hard to draw):
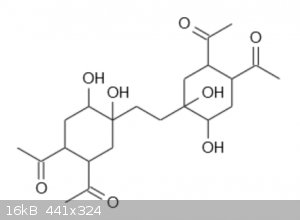
and the resulting polymer has a structure like this:
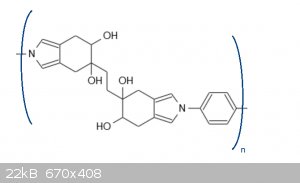
The hydroxy groups look like they may eliminate to form an isoindoline, but I do not think that this is likely because the cyclohexadiene ring in
isoindoline is not aromatic, so its formation should not be favorable. With the cyclopentadiene version this reaction is impossible due to Bredt's
rule.
Anyway, thoughts? Am I looking at the wrong thing entirely? I don't know that much about polymers, actually.
[Edited on 04-20-1969 by clearly_not_atara]
|