michalJenco
Hazard to Self
 
Posts: 50
Registered: 7-2-2019
Member Is Offline
|
|
Crystals in solution of butyric acid, ethanol and H2SO4
Hi fellow chemists.
I often prepare my Fischer esterification solutions in advance before the actual esterification. Today I noticed a small amount of colorless ~5mm long
needle-like crystals in a solution I prepared yesterday consisting of:
- 85g butyric acid
- 480g EtOH
- 90g 96% sulfuric acid
The solution was kept at room temperature and the number of crystals is very small, maybe 20-30 individuals in the whole volume. I also noticed
identical crystals in a similar esterification solution of hexanoic acid.
What can those crystals be? I searched for carboxylic acid - sulfuric acid adducts but didn't find anything.
[Edited on 5-1-2020 by michalJenco]
[Edited on 5-1-2020 by michalJenco]
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Very strange. I expect butyric acid and 96% H2SO4 are quite pure.
Maybe impurities in ethanol - what is used for its denaturation? Usually denatonium benzoate (Bitrex) but in very very very low concentrations (like
ppm or even less) and methyethylketone (usually 1%) - couldn't MEK undergo some acid catalyzed polymerisation?
|
|
|
michalJenco
Hazard to Self
 
Posts: 50
Registered: 7-2-2019
Member Is Offline
|
|
Yes, all reagents should be pure. Even if not, the two acids came from different suppliers. I distilled the EtOH from technical grade over KOH myself
so that should be fairly pure, too (no Bitrex or ketones).
I will have to do the butyric acid esterification again because my yield was terrible. I'll see if it happens again and I will try to take pictures if
it does.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Did you just do a simple distillation for the ethanol, or did you use a column? If you did a simple distillation and boiled it too quickly, it's
possible that some KOH was carried over as an aerosol into your receiving flask. It would have remained dissolved in the ethanol, so you probably
wouldn't have noticed, but it would have then reacted with the sulfuric acid to precipitate potassium bisulfate in your reaction mixture. Check the pH
of your ethanol, just in case.
|
|
|
michalJenco
Hazard to Self
 
Posts: 50
Registered: 7-2-2019
Member Is Offline
|
|
Damn, that sounds very reasonable Texium! I did fractionate with a 10cm Vigreux column but that might not have been enough to exclude 100% of the KOH.
Unfortunately all ethanol from that batch is spent now and a new batch was distilled using a 30cm column. If no crystals will form with this new
ethanol batch I'll take it as proof of your hypothesis. Thank you!
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
You can also use this to reduce droplets escaping by vapour:
https://www.kavalier.cz/lapac-kapek-sp223.html

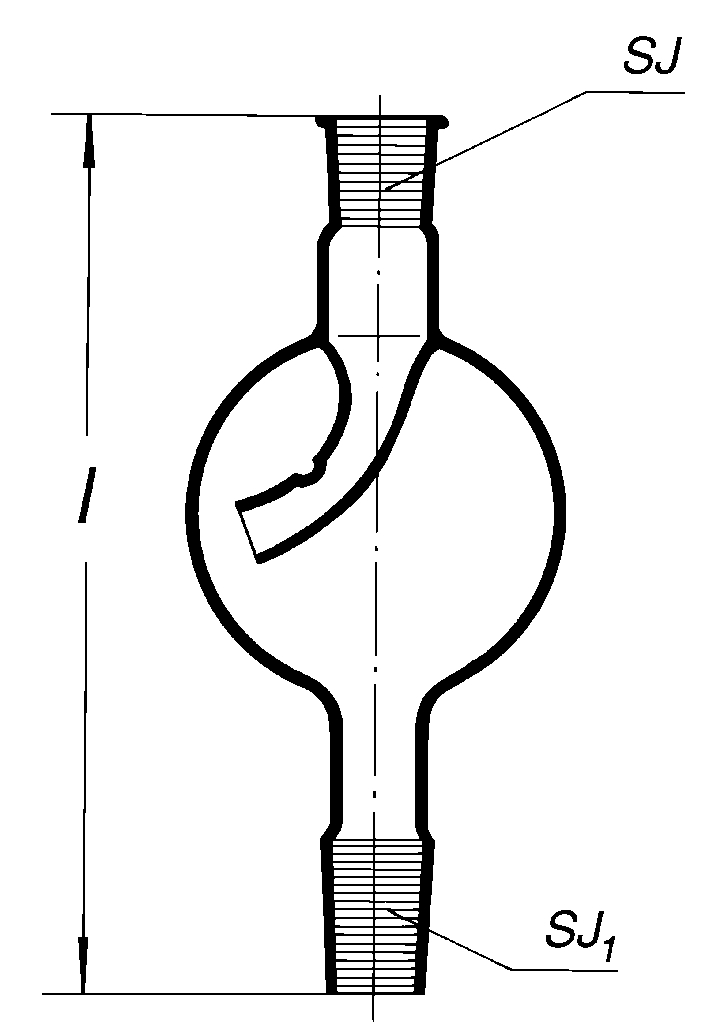
[Edited on 7-1-2020 by Fery]
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
so much H2SO4 which is just a catalyst... for consuming H2O to shift equilibrium towards product?
85g is it only a typo error and did you mean 850g ? ... or to use cheap ethanol in big abundance (10-fold excess) to shift equilibrium towards product
to save more expensive butyric acid ? ... but then a lot of H2O necessary to wash out the ethanol from the ester and solubility of ethylbutyrate in H2O is 4.9 mg/mL at 20 oC and very likely even higher if you did not dilute ethanol enough - how much H2O did you
use?
|
|
|