thalx
Harmless

Posts: 3
Registered: 12-11-2019
Location: Maryland Heartland, USA
Member Is Offline
|
|
Ammonium Persulfate etch waste --> CuCl2
Greetings,
Ammonium persulfate is sold as a PCB etchant. Once consumed, it is "waste". Once mixed, it will lose its etching ability in a short time frame (days
or a week or so). It works better hot, and barely at room temperature. There's scant information on how to dispose of it, so it tends to ...
accumulate.
Nurdrage has a video on making Copper Chloride etchant in three different ways, and one of the ways used Copper Sulfate as the starting ingredient. I
believe that Copper Sulfate is (a component of) the waste. Taking spent etchant waste, and transforming it into a renewable etchant is very
appealing! Additionally, Nurdrage indicates that the Sulfate ions are not a part of the etching process and don't interfere, so I jump to the
conclusion that it's very tolerant of any type of contamination. (likely incorrect on my part)
I took a small amount of the waste (slushy crystallized pale blue slurry) and under stirring with a stirbar I added small amounts of hydrochloric
acid.
The slurry turned a pale green, as expected. I added more HCl until there were no more blue crystals left... however, there is a lot of white
precipitate left behind. It amounts to about a third to a half of the beaker contents. White-ish precipitate on the bottom, bright green liquid on
top.
I tried a small strip of copper-clad PCB in the beaker, and while it did etch the copper, it wasn't as fast as I would have liked. It got through the
copper overnight.
Hindsight being 20/20, and me being a novice, I suspect a couple things. 1), I've neglected to consider what happens to the ammonium side of the
equation, and 2) my waste was not the deep blue of pure copper sulfate - rather it must have been a mix of copper sulfate with something else.
I believe that my "other stuff" is ammonium sulfate (in the original waste). After the HCL addition, perhaps I have some ammonium chloride, too?
Here's what I think happens in the original etching reaction:
(NH4)2S2O8 + Cu --> CuSO4 + (NH4)SO4
When we add HCL to the CuSO4:
CuSO4 + 2HCl --> CuCl2 + 2H+ + SO4-
And, considering the ammonium species,
(NH4)SO4 (?) + HCL --> (NH4)Cl + SO4-
-----
So I think I want to get rid of the ammonium compound, and perhaps by filtering the precipitate I can accomplish this. I've looked at solubility
ratings, and both Copper Sulfate and ammonium sulfate appear to have similar solubilities. If I've managed to convert (either, both, partially?) to
the chloride form, and have near identical solubilities, separation is perhaps difficult. I don't know how to determine the balance between Cu
conversion and NH4 conversion to Chloride - is there a way to tip the scales in one direction or the other?
Does anyone have any suggestions on how to proceed? To reiterate, I wish to convert my ammonium persulfate copper etching waste into CuCl2 etchant.
It seems feasible per my test batch, but is there a simple way to separate the ammonium from the copper - whether at a sulfate or chloride step,
and/or do I need to? If I reuse my CuCl2 etchant again and again, year after year, will I encounter any problems? Am I overlooking something, i.e.
what happens when mixed but unspent ammonium perchlorate solution "expires" and no longer etches? What's the decomposition results in that case?
Thank you in advance for any guidance.
-thalx
[Edited on 2020-2-21 by thalx]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The white material you obtained from the HCl indeed is NH4Cl. Just filter it and keep the green liquid. The NH4Cl can be discarded or flushed down the
drain (it is non-toxic).
The green liquid does etch your copper, but the reaction produces a very dark (almost black) copper complex, which is intermediate between CuCl and
CuCl2. Oxygen from the air replenishes the etching liquid. You, however, need to add acid every now and then to keep it active.
CuCl2 oxidizes copper. A very simplified reaction equation is this:
CuCl2 + Cu ---> 2 CuCl
CuCl in turn reacts with CuCl2 to form a nearly black complex CuCl.CuCl2 (in reality it is more complicated, but this describes the essence of this
reaction).
This CuCl-part of the black complex can be converted into CuCl2 again by adding acid and allowing oxygen from the air to be absorbed in the mix:
4 CuCl + O2 + 4 HCl ---> 4 CuCl2 + 2H2O
So, you get more and more etching liquid over time as more and more copper is dissolved from PCB's and more and more acid is added. You should try to
find concentrated HCl (30%). The stronger the acid, the better it works. Over time, your etching solution becomes better when its concentration of
CuCl2 rises and it is kept acidic.
The main disadvantage of this type of etchant ios that it gives off fumes of HCl and especially if you need to replenish it with oxygen from the air,
its container must be left open and unpleaant corrosive fumes are released, which may cause rusting of nearby tools. The replenishment best can be
done outside, overnight, while assuring that no animals can get in contact with the liquid.
|
|
|
thalx
Harmless

Posts: 3
Registered: 12-11-2019
Location: Maryland Heartland, USA
Member Is Offline
|
|
Thank you, woelen.
I forgot to mention that another big appeal to CuCl2 is another video by NurdRage whereby he reduces his volume of etchant by completely extracting
all the constituents with absolutely no waste. That's a green as chemistry can get! It takes multiple distillations, but he extracts water, HCl, and
electroplates out the copper. I think one is left with sulphuric acid, too. I don't know if this recycling process will work with a solution that
still contains sulfate ions, but I also won't have any distillation gear in the near future. But the fact that is is possible is another aspect of
what makes this very appealing. And I understand the toxicity of copper compounds to amphibians etc, so I will certainly not be flushing anything
down the drain.
I wanted to share some photos of the process.

1) - Ammonium Persulfate etchant, waste (solution self-dried and crystallized)

2) Beaker w/ waste, starting HCl addition (instant green color, assume CuCl2)

3) Beaker w/ converted waste, green CuCl2 liquid + white precipitate (assumed Ammonium Sulfate --> Ammonium Chloride)
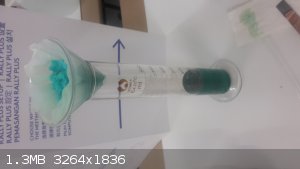
4) Filter setup, precipitate is actually blue, not white. Brilliant green liquid in bottom, even when I wash the filter with either water or HCl,
emerald green fluid drips. (Lighting bad, fluid looks dark in picture but is brilliant green in person) But it appears that the end product is the
same color as the beginning product. Rather confusing. But I guess this means that I haven't converted all the copper to CuCl2, perhaps I have been
too sparing in my HCl additions.
For my next step I will attempt to re-treat the .... (filter cake?) with more HCl and see if I can get more copper solution out of it.
-thalx
|
|
|
|