Hydrogen Fuel-Cell Cars
Here are some MS Publisher files showing a short article I recently had to write IMITATING the BBC 'Focus' magazine style, on Hydrogen fuel-cell cars.
The article features a brief introduction to the cells, some positive and negative aspects and a simple summary. Just to avoid any confusion, this is
NOT actually a BBC document, just something I had to produce in that style for my GCSE chemistry homework that I thought I'd share with you.
[The MS Pub files are difficult to open for many people, so I'll just upload the text content as well as leaving the .pub file available right at the
end.]
Naturally, the first step in studying hydrogen fuel cells, like any other topic, is to understand and appreciate how the system works and the science
behind it. Simply, the hydrogen fuel cell operates similarly to a battery. It has two electrodes, an anode, which is positively charged, and a
cathode, which is negatively charged, both of which separated by a membrane. Oxygen gas, passes over one electrode and hydrogen over the other. The
hydrogen reacts in the presence of a platinum catalyst that converts the hydrogen gas into negatively charged electrons (e-) and positively charged
ions (H+). The chemical equation for this process is as below;
H2 + [ Pt CATALYST] —> 2H+ + 2e-
The electrons produced flow out of the cell to be used as electrical energy. The hydrogen ions (positively charged atoms) move through the
electrolyte membrane to the cathode electrode where they combine with oxygen, obtained from the air, and the electrons, to produce water, given as;
4H+ + 2e- + O2 —> 2H2O
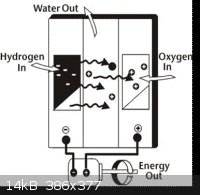
This process is shown in the last diagram below*, which illustrates the entry of the hydrogen fuel, the oxygen reactant and the exit of the energy
produced and water bi-product. Commercially, the hydrogen gas is produced via reforming of methane gas, CH4 , although small-scale electrolysis
sources are being considered and developed by scientists for the potential production of hydrogen gas domestically if the fuel cells grow in
popularity. The hydrogen fuel cell also has many advantages over standard petroleum or diesel engines; firstly, and perhaps most importantly from an
economic perspective, hydrogen fuel cells are 40% more efficient than standard engines—this equates to a better mileage that can be obtained per
unit of fuel used, and thus a saving in costs. Secondly, when hydrogen is reacted via combustion (burning) in air, its reaction with the atmospheric
oxygen produces only water as a product, which is obviously harmless for nature as it is part of the global water cycle that is essential for the
sustainability of our planet. This is as opposed to the harmful gases—namely sulfur dioxide, which is a major component of acid rain and detrimental
to human health, nitrogen dioxide—a toxic vapour that upon long-term exposure at concentrations above 40–100 µg/m3 may decrease lung function
and increase the risk of respiratory symptoms in humans, and carbon monoxide, a colourless, odourless, extremely poisonous gas—known as the
‘silent killer’ to name just a few, that come from the smoke exhausted from conventional engines. Thirdly, as they have a very simple design, mass
production is comparatively simple and thus they are cheaper to produce on an industrial scale. Hydrogen fuel cells, being similar to a battery in the
way the work but not in the sense that they cannot ‘run dead’ (other than by shortage of the hydrogen fuel itself), are also quiet to run, making
the journey in a car or other vehicle more pleasurable.
*Image courtesy www.making-hydrogen.com
Many people, however, are concerned about the aesthetic qualitites of cars featuring hydrogen fuel cells and other more environmentally-friendly
methods generating power for vehicle movement. These, however, are not usually actually an issue—a vast majority of modern cars, vans etc. featuring
these cells look like any other vehicle—the following images show the typical car fitted with such cells; pictured first is the new Honda FCX, the
latest development from Honda Motor Vehicles, the second is the Sport version of the same model and the third pictured is the famous Mini Cooper, but
re-fitted with a new hydrogen fuel cell.
{see below}
The stereotype of teenage boys having an interest in modern, fashionable cars, particularly those that are in some way powerful or otherwise superior,
was exploited; in a survey of 100 teenage boys aged between 14 and 19, when they were shown the above images only 3% could immediately identify that
they were fitted with hydrogen fuel cells, and despite this 89% agreed that they were aesthetically pleasing and would be proud to own them. The lack
of fossil fuels and their derivates required for these vehicles also helps the environment by helping to conserving these resources for the future,
however, the current main industrial method of procuring hydrogen, as aforementioned, is the reforming of methane gas with steam—and methane is an
alkane, originating itself from natural resources. The energy required for this process itself often also runs on energy generated via the burning of
fossil fuels, defying the point of using the hydrogen in the cars at the end (although, again as aforementioned, of course small domestic electrolysis
methods are being considered for the future.) This matter as a whole, however brings rise to some of the negative aspects of using hydrogen fuel
cells—there are of course the positive and negative aspects involved with any device, and these cells are no exception. Firstly, the leakage of
hydrogen gas has a detrimental impact on the stratosphere of the earth (cited from California Institute of Technology), and due to the above points,
in addition to the fact that generating hydrogen often requires more energy than is released when it is combusted, to replace all the vehicles would
require tenfold as much as is currently consumed. Secondly, storage is an issue because hydrogen is such a low density gas and the atom itself is very
small—allowing it to escape through the smallest of leak-holes in receptacles. The platinum used, although it is only a very small amount, is very
expensive as it is extremely rare and thus expensive, refining rock to produce the pure metal is very costly and time-consuming and the process is
often environmentally unsound. Thirdly, at the present time only 5 operational fuel stations for hydrogen exist in the UK, although a further 8 are
planned in conjunction with the development of the domestic electrolysis devices - this means that hydrogen is not very widely available, unlike
petroleum and diesel, meaning that individuals may be less inclined to purchase a hydrogen vehicle due to the threat of the appropriate fuel simply
not being locally available, short of the domestic apprati. Fourthly, although the cells themselves are comparatively cheap to produce, when the cost
of the other components of the car, including the hydrogen storage tank, connecting tubes/cylinders, safety valves, electronics, interiors and more
are considered, the total cost can become several times that of an equivalent-size standard petrol or diesel car—the cheapest begins at
approximately $75,000 USD (~£46,450 GBP) and some companies only offer them on lease—the cheapest this time being $600 USD per month (~£370 GBP),
or, over a 12-month period, $7,200 USD (~£4,460). It is therefore, upon analysing these facts and figures about hydrogen fuel cells, that
conclusions about the topic can be drawn. Firstly, it is obvious that they are better in use as opposed to standard petroleum or diesel engines at the
present time—despite not being an entirely renewable fuel, hydrogen is still better for our future sustainability and for the conservation of
natural resources. Hydrogen cells are also equally safe as petroleum or diesel engines, as the flammability and potentially explosive nature of
hydrogen is intentionally undermined by the complex network of monitoring sensors and safety valves included in vehicles fitted with the cells. Also,
although as previously mentioned the leakage of hydrogen gas can cause negative effects upon the stratosphere, the overall effect this has upon the
environment is minimal in comparison to the damage the use of conventional vehicle fuels can cause when the gases from the combustion chambers are
exhausted out of the system—the notorious carbon dioxide, a major component of global warming, surplus to the other vapours mentioned overleaf,
predominantley nitrogen dioxide, carbon monoxide, sulfur dioxide and more. It is for these reasons, therefore, that it would not be unreasonable to
predict that hydrogen fuel cell cars may be the solution needed to solve some of our energy problems in the future—but only at the arrival of
solutions to the main problems involved—notably a) the infrastructure issue, where hydrogen is not freely accessible to the average citizen, b) the
costs that are included, both from the car itself, maintenance, the cost of generating the hydrogen gas and more and c) the issues regarding the
potential economical impacts that could be caused upon the virtual monopoly that certain countries where oil is rich possess.
Attachment: chem h2 cells article.pub (1.3MB)
This file has been downloaded 794 times
[Edited on 28-4-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|