BJ68
Hazard to Others
  
Posts: 102
Registered: 12-3-2012
Member Is Offline
Mood: No Mood
|
|
Chemoluminescence of the Luminol-NaOH-DMSO System
Original Posting in German: http://illumina-chemie.de/chemolumineszenz-des-luminol-dmso-...
Equipment:
beaker glass (small)
2 x volumetric flasks (25 ml)
pipette (10 ml)
Gilson pipette (1000 µl)
balance
Chemicals:
DMSO (Dimethylsulfoxide) CAS: 67-68-5
Luminol CAS: 521-31-3
NaOH CAS: 1310-73-2
fluorescein sodium salt CAS: 518-47-8
Attention: DMSO can act as a carrier for substances with a molecular weight up to 3000 unit, directly through the
skin
Preparation:
Put about 0.75 g NaOH in 2 ml of water and dissolve it. Fill the two volumetric flasks with 10 ml DMSO and add in one of these flask a small amount of
fluorescein sodium salt plus about 50 mg of luminol in every flask.
Now add 200 µl of the NaOH-Solution and shake the closed flasks. After while you will see a nice glow.
Explanation:
Sodium hydroxide reacts in this DMSO-Water solution* with luminol to the dianion of luminol, which reacts with the oxygen in the flask and makes a
endo-peroxide, which degrades under the emission of light.
*= The paper "A new, long-lasting luminol chemiluminescent cold light" J. Chem. Educ., 1970, 47 (7), 519 (DOI see Literature) says, that rising
water content (over 5%) will quench the reaction and if the concentration is over 20%, the reaction will be completely inhibited. If you adjust the
luminol concentration to 10 mg/ml the oxygen in the flask will be consumed very quickly and only pure nitrogen will be left over.
Here is the mechanism:
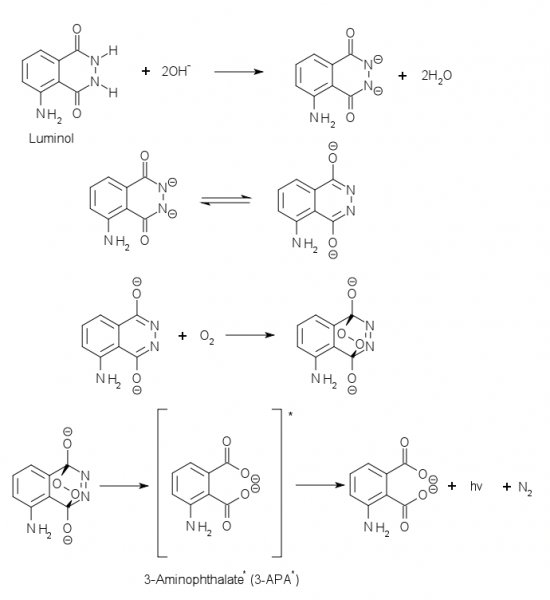
Source (Accessed: 4. February 2013)
Waste-Management:
In Germany there are places where you can get rid of chemical hazardous waste, without charge. So investigate your local possibilities, for this kind
of waste (Solvents, non halogenated)
Pictures:
The Chemicals:
Sodium hydroxide Solution:

10 ml of DMSO:

Weighted Luminol:

Luminol-DMSO-Solution:

Glow of pure Luminol:

Glow of Sodium-Fluorescein-Luminol-DMSO-NaOH-Solution:

Comparison of both solutions:

Literature-Sources:
Experiment derivated from
A new, long-lasting luminol chemiluminescent cold light
Secondary source (in German):
Chemolumineszenz des Luminols
(Accessed 4. February 2013)
Mainpage with experiments
Remarks
At a scout camp I made a bottle with about 400 ml DMSO Solution and used very approximately amounts (few gram NaOH, a spatula tip of luminol and
fluorescein sodium and about 8 ml of water) and it worked, too. There was a delay of 15 seconds.....but after shaking the whole bottle was glowing.
IMPORTANT: Use a good PTFE-Septa-Cap, because the kids will put the bottle anywhere...and I mean really ANYWHERE.
Bj68
[Edited on 4-2-2013 by BJ68]
|
|
|
Cloner
Hazard to Others
  
Posts: 150
Registered: 7-12-2004
Member Is Offline
Mood: apocalyptic
|
|
Coincidentally, I have just finished making a sizeable amount of luminol. Unfortunately, I cannot access the JCHEMED article. I would be very
interested in a means of making luminol glow for a longer time.
Can you compare the effect of luminol using the aprotic solvent system with the basic peroxide system? If this bottle went from hand to hand, it seems
like your method is superior.
|
|
|
|