| Pages:
1
..
3
4
5 |
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Reading this topic I remembered a study I've saw, telling about formylation of phenols with 80% yields.
They used anhydrous MgCl2, EtN3 and Paraformaldehyde in THF at reflux and this is described at orgsyn.
From the salicylaldehyde formed is easy to get cathecol by using H2O2 in basic media. This is also described at orgsyn.
The both synthesis is attached below:
Attachment: formylation of phenols - MgCl2 + Et3N + paraformaldehyde in THF.pdf (535kB)
This file has been downloaded 524 times
Attachment: CATECHOL from Salicylaldehyde or guayacol.pdf (168kB)
This file has been downloaded 625 times
[Edited on 13-3-2018 by Chemi Pharma]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Does anyone know the reaction mechanism for this overall reaction?
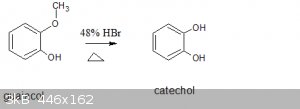
Attachment: catechol chemsketch equation.sk2 (2kB)
This file has been downloaded 936 times
[Edited on 1-4-2018 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
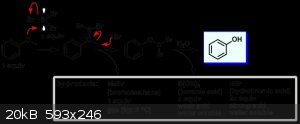
Protonation of the phenol methyl ether and substitution with bromide giving methyl bromide which off gasses, and cathechol. Pretty similar to phenol
demethylation by boron tribromide just under much harsher conditions.
http://commonorganicchemistry.com/Rxn_Pages/O_Demethylation/...
Edit: on clicking some more here is the actual mechanism :
http://commonorganicchemistry.com/Rxn_Pages/O_Demethylation/...
[Edited on 1-4-2018 by Sigmatropic]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks very much!
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I was actually just reading about this reaction in Preparative Organic Chemistry (in the SM library) yesterday. Apparently, a very wide range of acids
can be used, but boron tribromide will dealkylate phenyl alkyl ethers if added at dry ice temperatures and allowed to warm to room temperature.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Preparation of Catechol from Guaiacol 8/30/18
This write-up describes a 0.018 scale preparation based on the procedure in OrgSyn (ref a).
1. Chemicals
150 ml guaiacol
279g 48% HBr
300g methanol
500 ml commercial toluene
2. Apparatus
a. 40cm 24/40 Hempel column, broken glass packing
b. 500ml 2-neck RBF w/Claisen adapter, 24/40
c. condenser, 24/40
d. separator, custom made by Eagle Lab Glass
e. assorted 8mm and 6mm glass tubing
f. 150°C thermometer
g. distillation adapter and vacuum adapter, 24/40
h. 1000ml 24/40 rbf
i. overhead mechanical stirrer
j. Bunsen burner, support ring, and wire gauze.
k. fume hood
l. heat gun
m. mortar & pestle
3. Procedure
The purchased guaiacol and homemade 48% HBr were added to the 500ml pot. The mixer was turned on to 500 rpm and heating applied by bunsen burner.
Heavy insulation was applied to the pot (fiberglass blanket and Al foil). Sufficient heat was applied to bring the vapor at the head of the column to
95°C. As the vapor was condensed it ran into the separator, returning guaiacol to the pot. Methyl Bromide in the vapor was absorbed in an
ice-cooled methanol filled flask. Water overflowed from the separator to a waste receiver (note a). When no more guaiacol was returning to the pot
the temperature was brought up to 100°C for an hour. The distillation was then terminated.
Show movie here (YouTube will not let me post this movie as they claim I have already posted one like it. However, I can not find this.)
The pot contents were then extracted 3 times with 167ml toluene. The combined extracts were then
filtered obtaining catechol crystals contaminated with a slight purple colored material reminiscent of potassium permanganate. The crystals were then
subjected to vacuum distillaion (30mm Hg) using a short path condenser and a heat gun (mp = 105°C).
The10.6g of catechol was then ground to a powder (note b). This product was not purified further as a melting point of 104°C was obtained.

(toggle to invert picture)

The toluene extract had a translucent yellow color and was distilled obtaining perfectly clear toluene. The pot residue contained a further 1.0g of
catechol. This was recrystalized from toluene giving a mp of 105°C.
4. Yield
The yield was a very low 11.6g for a %yield of 7.7%. The very small scale of thei preparation compared to that of OrgSyn (76% yield) likely was a
major factor.
5. Conclusions, etc
This procedure produces high purity catechol at a low yield. Comments, questions, and recommendations are cheerfully solicited.
6. Notes
a. The separator was too large for this application. One 1/3 this size would have been better.
b. Molten catechol poured into the mortar should be ground as it solidifies. When solidified it is extremely hard.
7. Reference
a. Organic Syntheses, Vol 3, p.28 (1923), Catechol [Pyrocatechol] from Guaiacol by HT Clarke and ER Taylor.
[Edited on 30-8-2018 by Magpie]
[Edited on 30-8-2018 by Magpie]
[Edited on 30-8-2018 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Very nice write up! I wouldn't expect the low yield is a result of small scale, 150 mL of guaciol is already on a decent scale IMHO. But for a
preparation at 18% scale that seems to be too little toluene, orgsyn calls for three 1500x0,18=279 mL portions. Also I believe there is a sentence
missing or did the extracts just start to crystallize spontaneously? That would be an indication you're leaving quite some material behind in the aq.
Phase.
[Edited on 31-8-2018 by Sigmatropic]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you for your constructive comments. If I ever do this again I will incorporate your suggestions.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
Nice to see your lab work again. You have been away a while.
I think Sigmatropic is correct in that you may be leaving behind product in the aqueous layer. According to a Google search, catechol has high water
solubility (430g/liter) but not so much in cold benzene which may carry over to toluene solubility. Continuous extraction may be in order or perhaps
steam distillation (arg) or a different extraction solvent. Do you have the means to follow the reaction or the extraction by tlc? I can't think of
any reason that the reaction as run should give a poor yield. The purple material is just probably oxidation product of the catechol and you imply
that it is just a small amount.
Anyway, I hope you did not sewer the aqueous layer. We were always told to look at everything and get the mass balance when things went awry.
Nice experiment - real chemistry.
AvB
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The correct scale was 0.183, as you have pointed out. Therefore I should have used 3x 274ml toluene, not 3x 186ml. I did sewer the water. Having
the huge apparatus set up in my hood for 8.5 months while I got the stepper motor mixer running I was impatient to get this experiment finished.
But thanks for the compliments, both of you.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I too am glad to see you're back in the lab Magpie. I have several queries/suggestions to make, and whilst they are of little use to you now that the
reaction mixture has been disposed of, they may provide benefit on a subsequent run or an attempt by another member.
It looks as if the "reaction phase" of the experiment proceeded as described by the OrgSyn authors, and I'm confident that if you had checked the
reaction by TLC or other means you'd have seen that conversion was good. As with any reaction that underperforms/fails, the starting materials and
reagents should be scrutinised, although I'm sure that, given your thorough nature, the Hydrobromic acid had been previously titrated and the guaiacol
assessed (crystalline?) or otherwise purified.
All of this suggests that the difficulty is with the workup. The catechol as AvBaeyer mentions is very soluble in water at ca 430g/L; it is
uncertain how this translates to the depleted HBr solution but I suspect it is considerable. This, coupled with the low solubility of catechol in
toluene at room temperature, means that it is essential that the extractions take place at elevated temperature (85 to 95 *C) as specified in
the OrgSyn preparation.
Now, you don't mention how this was performed, but the way I would do it would be to add the first portion of toluene to the flask, add a condenser,
and heat the mixture with vigorous stirring up to the desired temperature. Stirring maintained at this temperature for a short duration ( 5 mins), and
then the mixture transferred into a sep funnel and separated whilst hot. The aqueous would be returned to the flask, and the extraction repeated twice
more with fresh portions of toluene. This way you can be sure that the extraction is performed at the desired temperature as both the aqueous and
solvent are heated.
Also notice that despite these extractions at elevated temperature, the authors only obtain approximately 50%th yield, again indicative of the high
aqueous solubility of catechol. Additional product (up to ca 85%th combined) is isolated by re-working the aqueous stream.
Now, all this isn't to say that you can't use a different solvent for the extraction, and if the catechol has good solubility in the solvent then the
extractions can be performed at room temperature. Consideration should be given to the strongly acidic, aqueous nature of the reaction mixture. The
OrgSyn authors mention that carbon tetrachloride can be used in place of toluene to advantage, but do not comment on the solubility of catechol, and
whether it is good enough to perform the extractions at room temperature. If an alternative solvent is found, then the crude product can still be
recrystallised from toluene, or distilled/sublimed, for purity, although the OrgSyn authors appear to have used toluene for a more streamlined
isolation/purification process.
Regardless, good work and I'm glad you managed to isolate some good purity material, even if the yield wasn't what you was expecting. There's nothing
worse than a total write-off.
|
|
|
| Pages:
1
..
3
4
5 |