Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Bis-(5-tetrazolyl)amine Preparation
Preparation of Bis-(5-tetrazolyl)amine
5-aminotetrazole is now a well known and fairly accessible nitrogen rich heterocyclic but the di-substituted amine analogue is less well known.
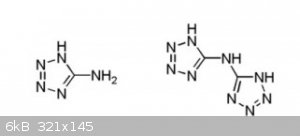
A comparison between the structures of bis(5-tetrazolyl)amine (Right) and 5-aminotetrazole
There are two principle routes to this compound, one from cyanuric chloride and the other from sodium dicyanamide; other methods are summarised in the
reference cited below but are either subtle variations of these method or via the addition of a tetrazolyl group to 5-aminotetrazole using cyanogen
bromide and sodium azide. The former method is due to Nedel'ko et al. 2005 (1) and is a four stage synthesis with an overal efficiency of about 20%
and proceeds via a rather sensitive bis-azido triazine. While of interest this synthesis is rather low yielding and potentially hazardous.
The more direct and for me preferred route is via sodium dicyanamide. This procedure originally appeared as a US patent 5468866 (2) with a slightly
revised procedure published by Klapötke et al in 2008 (3). In US 546866 Highsmith et al reported yield up to 80+ % using sodium dicyanamide, sodium
azide and either ammonium chloride (impure product) or boric acid (purer product and variable yields). The method of Klapötke et al is basically
similar except that it uses the slow addition of hydrochloric acid to liberate free hydrazoic acid. These methods are essentially similar to the
method of producing 5-aminotetrazole from cyanamide and dicyandiamide described elsewhere on the SM form in posts by Engager.
Amongst a collection of chemicals I acquired recently I found a 100g jar of sodium dicyanamide and so decided to try the method of Klapötke et al. I
used their procedure with only very slight modification to take into account the lower concentration of HCl in my hydrochloric acid.
Experimental
The equation that roughly represent the synthesis described below is:
2NaN<sub>3</sub> + NaN(CN)<sub>2</sub> + 3HCl + H<sub>2</sub>O → 3NaCl +
NH(CN<sub>4</sub>H)<sub>2</sub>H<sub>2</sub>O
In 3 neck 3L r.b. flask were placed 44.51g of sodium dicyanamide and 65.01g of sodium azide. To these were added 400ml of 95% ethanol and 400ml of
water and the flask swirled to disperse the solid which do not dissolve at this stage.

Initial reaction mixture after addition of all the hydrochloric acid giving a clear solution.
The flask was place in a heating mantle, a reflux condenser and a large (600ml) dropping funnel added and the flask heated almost to boiling. From the
dropping funnel were added 155ml of 30% HCl, diluted with 500ml of water, over a period of 5 hours while maintaining a steady gentle reflux. After the
addition the flask was refluxed for a further 43 hours (48 hours in total), cooled a little and poured into a 2 L beaker; a white precipitate beings
to form almost immediately so 85ml of 36% HCl were added immediately in one go while the solution was still hot. The solution was stirred and allowed
to cool to room temperature then chilled in the fridge until the temperature reached 4°C. A large amount of very fine, pure white, BTA monohydrate
separated quickly upon acidification.

Precipitating BTA monohydrate after the addition of the concentrated hydrochloric acid.
When cold the precipitate was recovered by filtration at the pump (Note 1) and washed with a little cold water and then 95 % alcochol and sucked as
dry as possible. The precipatate is very fine and filters slowly. The filter cake was broken up on a large watch glass and dried to constant weight on
the back of an operating oven (40-45°C) for 24 hours, the yield was 84.02g of crude bis-(5-tetrazolyl)amine monohydrate. This is almost theoretical
yield and it is suspected that it is either not fully dried or contaminated with sodium chloride.
After some small scale experiments it was found that the best means of purification is to recrystallize the material from 3% hydrochloric acid, about
40ml per gram of BTA monohydrate being required. Since this involves the manipulation of more than 3 L of acid it was decided to carry out the
recrystallization in stages using just 1 L of 3% hydrochloric acid.
100ml of 30% HCl was dilute to 1 L in a 2 L beaker and 24g of the crude BTA added. The temperature was gradually raised until most of the material had
dissolved, this required more than 30 minutes at over 90°C. Filter hot through a pre-heated Buchner funnel and place in a large beaker to cool and
crystallise. When cooled to room temperature filter in a smaller Buchner funnel to recover the pure white crystalline BTA monohydrate which now
filters readily. Remove the filtrate and return to the 2 L beaker and then wash the filter cake first with ice cold water (50-70ml) and then with 95%
ethanol (Note 2) and suck as dry as possible and place on one side to dry as before.
To the filtrate were added another 20g of the crude BTA and the acid reheated until it had practically all dissolved and the above filtration and
crystallisation repeated. Repeat this process for each of the two remaining 20g portions of crude BTA. The four fractions were dried and combined. The
yield was 67.00g or 78.3% yield of a pure white free flowing crystalline powder.
On heating it loses water slowing becoming a fluffy powder but it does not melt only decompose quietly. It is soluble in dilute sodium hydroxide
solution and this solution gives a white precipitate with dilute lead nitrate solution. Once dry the lead compound deflagrates suddenly with a "phut"
sound and a pink flame when heated but is otherwise surprisingly insensitive. Dilute copper sulphate with the sodium salt gives a deep green
colouration.
Note 1) An 11cm Buchner funnel and Whatman 1 paper proved adiquate.
Note 2) Remove the acid filtrate before washing so as not to dilute it; furthermore keeping the washing seperate facilitates the recovery of the
alcohol (over 250ml were used in the 5 washings.
References:
1) The Synthesis and Thermal decomposition of Ditetrazol-5ylamine; V.V. Nedel'ko et al.; Russian Chemical Bulletin, International Edition; v54, 7,
pp1710-1714; 2005
2) Methods for Synthesizing and Processing of Bis-1,2H-(tetrazol-5-yl)amine; Highsmith et al.; 1995; US patent 5468866
3) Bistetrazolylamine, Synthesis and Characterisation; J. Mat. Chem.; Klapötke et al.; 2008; vol 18; pp5448-5458
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Great synth.! I don't know much about organics, so I couldn't say much on how it's written, but looks great!
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Very nice writeup. My interest in explosives doesn't go much further than interesting structures, and the work here on scimad on tetrazoles has always
been an interesting read. Now, we need a writeup on Sodium Dicyanamide from OTC materials!
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@UC235, actually my interest in these types of tetrazole and triazole compounds is in their ability to act as ligands. I have been too busy recently
and away from home much of the time so I haven't been able to continue this work.
I obtained a small amount of sodium dicyanamide from a friend along with various other chemicals and though very old appears perfectly serviceable.
Making this compounds looks tricky. There are a significant number of patents concerning this preparation but most require substances like cyanogen
bromide and sodium cyanamide, though some use free cyanamide. I have recently cracked the free cyanamide problem and can now prepare it easily and
cheaply from industrial calcium cyanamide but I can't see a way round the cyanogen halide.
Dicyanamide salts appear fairly stable and are usefull for preparing numerous amino-heterocyclic too. The barium salt reacts with hydrazine sulphate
to give 3,5-diamino-1,2,4-triazole. The sodium salt react similarly but the sodium sulphate is a little harder to seperate.
[Edited on 24-3-2015 by Boffis]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by Boffis  | @UC235, actually my interest in these types of tetrazole and triazole compounds is in their ability to act as ligands. I have been too busy recently
and away from home much of the time so I haven't been able to continue this work.
I obtained a small amount of sodium dicyanamide from a friend along with various other chemicals and though very old appears perfectly serviceable.
Making this compounds looks tricky. There are a significant number of patents concerning this preparation but most require substances like cyanogen
bromide and sodium cyanamide, though some use free cyanamide. I have recently cracked the free cyanamide problem and can now prepare it easily and
cheaply from industrial calcium cyanamide but I can't see a way round the cyanogen halide.
Dicyanamide salts appear fairly stable and are usefull for preparing numerous amino-heterocyclic too. The barium salt reacts with hydrazine sulphate
to give 3,5-diamino-1,2,4-triazole. The sodium salt react similarly but the sodium sulphate is a little harder to seperate.
[Edited on 24-3-2015 by Boffis] |
So do you have any results on their usage as ligands?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The usage as ligand will be quite tricky because you have 3 H atoms that are quite acidic...so there will be competition between salt formation and
complexation.
This is seen with dimethylglyoxime (where one oxime group act as an acid and the second one as a complexant) and with acetylacetone (in
acetylacetonate, one ceton (keto) group turns into an enol group and act as an acid while the other keto group serve as complexant).
So one may maybe get both effects? What might be good
BTAA + Ni2(CO3)3 --> NiBTAA (Nickel (III) bistetrazolamin-ate) + 3 H2O + 3 CO2(g)
NiBTAA + 2 BTAA --> NiBTAA. 2 BTAA
(if BTAA coordinates as tridentate via two N doublets from tetrazoles (one from each) and from NH bridge)
NiBTAA + 3 BTAA --> NiBTAA. 3 BTAA
(if BTAA coordinates as bidentate via two N doublets from tetrazoles (one from each))
On another aspect,
I wonder if BTAA will react with chlorine to make BTAA N-chloride and then with silver cyanide to make BTAA N-cyanide (A potent explosive and
superfuel!); the later would then be reacted with hydrazoic acid (NaN3/HCl) to provide a Tris-5-TetrazolylAmine (TTAA)...another potent explosive and
superfuel.
TA-NH-TA + Cl2 --> TA-NCl-TA
TA-NCl-TA + Ag-C#N --> (TA-)2N-C#N + AgCl(s)
(TA-)2N-C#N + HN3 --> N(TA)3
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi PZ, my apologues for taking a while to respond but my computer died rather suddenly just as was about to go on holiday. I´m up and running again
now though.
In the paper by Klapotke et al referenced above they actually state that the tri-(5-tetrazolyl)amine had not been prepared (in 2008) so I would be
interested to see any references you have to it preparation. They describe the stepwise methylation of the bis-(5-tetrazolyl)amine in which the
imino-hydrogens on the tetrazole ring were replaced first and then the hydrogen on the amine. I thought about trying to block the tetrazole hydrogens
by reacting them with t-BOC or Diethylcarbamyl chloride first and then de-protonating the bridging amine with a strong base before reacting with
cyanogen bromide to give the bis-(tetrazolyl)cyanamide. This would then be reacted with azide+acid to generate the third tetrazole ring. The problem
with this route is the number of unobtainable compounds.
I think your route sounds marginally easier, at least from the point of reagent acces, but I think you would also suffer from the need to protect the
imino hydrogens on the tetrazole rings. Also is it posible to turn a strongly acidic imino hydrogen into a chloramine?
I have yet to investigate the salt and complex forming abilities of this compound but a few quick test-tube reactions suggest from the colour that
copper and cobalt form complexes. I did a bit of research and found that the bis and tris 2-pyridylamines have been studied and form complexes with
interesting properties but these compounds have no imino hydrogens on the heterocyclic ring.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Thank you Boffis for your return  on this. on this.
Great to read that complexes seems to form in preliminary tests.
I have no documents or reference on this (I wrote "I Wonder if")  so I was just
writing theorical idea based on practical knowledge. so I was just
writing theorical idea based on practical knowledge.
Halogenation of an "acidic" imino should not be a problem. Although storage might be unadvisable
1°)Urea does form a chloramine and a dichloramine despite the strong electro-withdrawing effect of the C=O.
NH2-CO-NHCl
ClNH-CO-NHCl
2°) Also NH2Cl turns into NHCl2 and NCl3 while each added halogen atom increases the acidity
3°)Axt has tried chlorination of NH-nitramine to get chloronitramines. NH-nitramines are very acidic!
R-NH-NO2 -halogenation-> R-NCl-NO2
He postulated the possible use of those to get to alkyl dinitramides
R-NCl-NO2 + AgNO2 --> R-N(NO2)2 + AgCl
True that it is hard to tell if halogenation will happen at the (het)-NH-(het) bridge or at the -NH- in the tetrazole rings (or at both places). But
anyway the resulting molecules will be very interesting.
1°)If the imino-tetrazole do react:
TA-NH-TA + Cl2 --> Cl-TA-NH-TA-Cl
Cl-TA-NH-TA-Cl + 2 Ag-C#N --> (N#C-TA-)2NH + 2 AgCl(s)
(N#C-TA-)2NH + 2 HN3 --> HN(-TA-TA)2 (bis-di-tetrazolyl-amine)
2°) If all imino do react
TA-NH-TA + Cl2 --> Cl-TA-NCl-TA-Cl
Cl-TA-NCl-TA-Cl + 3 Ag-C#N --> (N#C-TA-)2N-C#N + 3 AgCl(s)
(N#C-TA-)2N-C#N + 3 HN3 --> TA-N(-TA-TA)2 (bis-di-tetrazolyl-aminotetrazole)
The extension of the procedure by iteration would lead to:
N(-(TA-)n-H)3 from N(TA)3
HN(-(TA-)n-H)2 from bis-di-tetrazolyl-amine
(H-(-TA)n-)N(-(TA-)n+1-H)2 from bis-di-tetrazolyl-aminotetrazole
An alternative pathway to TTAA would be to start from aminotetrazole:
H2N-C(N4H) -halogenation-> Cl2N-C(N4H)
Cl2N-C(N4H) + 2 Ag-C#N --> (N#C-)2N-C(N4H) + 2 AgCl
(N#C-)2N-C(N4H) + 2 HN3 --> N(-C(N4H))3
[Edited on 20-4-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Philou, how did you get on with the references?
I have been looking into this further. Thinking about your last idea first, do you think this is possible given that hypochlorite tends to oxidize
these sorts of amines to azo compounds, see nematic´s thread http://www.sciencemadness.org/talk/viewthread.php?tid=14350#... ?
The cyanogen halide route looks better but having re-read Klapotke´s paper again I am pretty convinced that it will be necessary to block the imino H
on the tetrazole rings as I discussed above. In the Klapotke paper he discribes the selective methylation of these imino H´s with methyl iodide and
alkali. For me, in my hunt for ligands I don´t think this will be a problem and indeed may be a bonus turning the compound into a monobasic acid
ligand. For you this may be more problematic so I wonder if you could acetylate the two imino H´s with acetic anhydride, react the remaining
secondary amino H with cyanogen bromide and then de-acetylate during the acid recrystallisation at the end.
Alternatively could you use t-Boc to block the imino H´s or does it only work with primary amine? I also thought about carbamoyl groups, I am looking
into this route for 1,2,3-triazoles but in this case the carbamoyl (H2N-C=O- group) is attached to the azide group from the start (via carbamoyl azide
from semicarbazide). This group is easily removed.
Another avenue that I am looking at is to prepare the methylated BTA as per Klapotke´s route and then react the product with 2-chloropyridine and a
base such as triethylamine. I have found references to similar procedures for other pyridyl- substituted amines. It appears that the 2-chloro group in
pyridine is quite reactive. This would give me a neutral bis-(5-tetrazolyl)-2-pyridyl amine ligand. This raises the question of how reactive would the
5-chloro or bromo tetrazole be under these circumstances? These compounds are available from 5-aminotetrazole via a route essentially similar to that
used to prepare 5-nitrotetrazole (there is paper in German by Stolle giving details Berichte v62 p1118 (1926)).
So routes to try!-
1/ acetylate amino hydrogens, react product (as Ag or Na salt)with 5-halotetrazole, de-acetylate
2/ acetylate as above, react sodium salt with Cyanogen bromide followed by Na azide+ammonium chloride
3/ acetylate as above, react with hypochlorite, then with silver or copper I cyanide then azide+ weak acid as above
If you are not worried about losing the imino H´s then di-methylation looks like the way to go.
Your ideas about blanket chlorination raise the possibility of yet another very indirect route. I was curious about tetrazole N-chloro and N-cyano
derivatives so I did some research and could not find anything on tetrazole chlorimines or N-cyano derivatives. If it is possible to introduce N-cyano
groups to replace the imino hydrogens these may be a surragate for the carbamoyl groups I speculated on above since they are likely to be hydrolizable
to a transient formyl group which should then be removable too. So it may be possible to form a bis-(N-cyanotetrazolyl)amine, convert this to the
bis-(N-cyanotetrazolyl)-tetrazolylamine by one of the routes above and then hydrolyse the cyano groups. However, there is considerable evidence of
degradation of the tetrazole ring by hypochlorite and hypobromite (see Thiele Annalen v303 p57 (1898))
This route is highly speculative inview of the lack of published references to either tetrazole ring chlorime or N-cyano derivatives and also inview
if my comments above re hypohalite degradation. However, it gave me another idea, to try and generate the bis-(N-methyltetrazolyl)-tetrazolylamine
from the dimethylated BTA described by Klapotke and them oxidize the methyl groups with potassium permanganate as Bladin had done in his original
discovery with the N-phenyl group.
So the route I think I am going to try is to prepare the dimethyl BTA as described by Klapotke; convert it to the monosilver salt and react this with
5-iodotetrazole (desribed in the paper by Stolle ref above) and finally remove the methyl groups by oxidation the alkaline permanganate.
I´m only missing one thing now.... TIME
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
From the tread References: Wanted References and Needed Translations (9)
Quote: Originally posted by Boffis  | Hi Philou,
When you have had a read at these let me know what you think. Could I block the two imino hydrogens on the tetrazolyl rings with say acetyl groups
before reaction the bridging imino with say cyanogen bromise? Lets discuss in the main thread.
|
Thank you Boffis for the reference documents you posted as reply to my request of documents.
In the link you posted the hypochlorite indeed do oxydise heterocyclic amines but the conditions are quite harsch (boiling water temperature
hypochlorite).
You should maybe take a look at Axt tread about halo-amines...those are powerfull explosives but they are made with basic amines like CH3-NH2,
H2N-CH2-CH2-NH2 and the explosive power comes from the hydrogen contained (burned by the halogen to make HCl), the density of the compound (because of
the halogen) and because the parenthood with overly sensitive NCl3, NBr3 and NI3 (NH3-NI3).
Such compounds doesn't like to be heated.
From the documents you provided to me, it is clear:
-that Br-C#N does react on the external NH2 of aminotetrazole and not on the imino from the cycle.
But nothing is seen about the reaction of Br-C#N on BTA 
-that NaOH and CH3-I react on the imino groups from the cycles of BTA and not with the bridging imino NH...the bridging imino only methylates upon
further reaction with NaOH and (CH3)2SO4 and heat.
Since Br-CN has no special affinity for the imino of the ring in aminotetrazole there is a chance it is the same for BTA (the acidity of those remains
about the same) and this may give a chance that its afinity will be bigger for bridging one.
All your pathways looks good and reliable based on theory and practice.
Halo-C tetrazole are indeed formed via diazotation of aminotetrazole...just like with aromatic amines to replace the amino by a halogen atom. Those
halo-C-tetrazole will display the same kind of reactivity as cyanuric trihalide...they are acid halides.
As such they may hydrolyse to form an enolic acid (hydroxy-C-tetrazole  HO-CHN4
and maybe this may give an anhydride like diphenyl ether from phenol --> tetrazolyl ether N4HC-O-CHN4 )...tetrazolenolic acid if stable and its
salts should be very interesting. HO-CHN4
and maybe this may give an anhydride like diphenyl ether from phenol --> tetrazolyl ether N4HC-O-CHN4 )...tetrazolenolic acid if stable and its
salts should be very interesting.
The halogen is reactive and should easily be replaced:
-by amine (like melamine from cyanuric chloride)
-by methylamine or other amines
-by azide (like cyanuric azide from cyanuric chloride)
-by acetate (from acetate salt thus forming a mixed anhydride)
-by alcoholate (forming an ether (actually more like an ester) like anisole (methylphenol))
...
and maybe with nitroformiate to form trinitromethyl-C-tetrazole.
The methylation and further oxydation by permanganate is very likely to work because as you know from your triazole synthesis based on benzotriazole
oxydation, heteroaromatic rings are very resistant to oxydation.
If there are troubles you may also work with formylation instead of acetylation or with chloroformic esters (Cl-CO2-R).
[Edited on 12-5-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|