frogfot
Hazard to Others
  
Posts: 212
Registered: 30-11-2002
Location: Sweden
Member Is Offline
Mood: happy
|
|
Introducing an extra carbonyl group
Found some very interesting reaction in my course book:
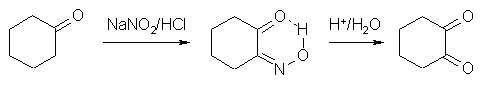
The mechanism is simple. Here we first form an enole that reacts with NO+ and some rearrangement takes place. In second step the oxime (middle
compound) is hydrolysed to give second carbonyl group.
The fun part is that no "exotic" chems are needed.. I've searched my old org books for synthesis of 1,2 cyclohexadione (third compound
in scheme above) but found only one reference (Org. Syntheses, vol 26-32) that uses cyclohexanon and SeO2..
So, I assumed this type of nitrosation to be kinda new..
The first thing that came to mind is to make this reaction with acetone to get OHC-CO-CHO
Any references to theese types of reactions?
Btw, one of my references did OHC-CO-CHO by oxidising phorone by ozone, whereby acetone peroxide formed as byproduct.. must be a scary synth 
|
|
|
Kinetic
Harmless

Posts: 45
Registered: 31-12-2004
Member Is Offline
Mood: fine
|
|
Nitrosation
This is an old but good route whose precedent has been set by quite a large amount of literature in the past. I don't have much time at the
moment but I would advise you to look at Patent US2248035 to begin with. I recently suggested this route to Organikum as part of a route to prepare glycols which can be rearranged to
phenyacetones. But this really depends what you have in mind for this transformation. Not everyone is interested in making phenylacetones.
A nitrite ester is often used instead of the sodium salt. For our purposes, of course, the use of the readily available salt is preferable. I can
probably find some more literature references if you are interested. The route is also used to prepare phenylpropanolamine (and analogues) by omitting
the hydrolysis of the intermediate alpha-oximinoketone produced by the nitrosation of propiophenone, and instead reducing it. Dr. Dave
Nichols has used nitrite esters in syntheses of analogues of MDA in the past.
|
|
|
frogfot
Hazard to Others
  
Posts: 212
Registered: 30-11-2002
Location: Sweden
Member Is Offline
Mood: happy
|
|
Cool, thanks for the patent. I'm sure that their general procedure for oximinopropiophenone can be applied to other ketones. Although I'm
not sure what's purpose of NaCl.. is it suppose to salt out the product? Then why not add it in the end..
I had no particular reactions in mind.. just thoat to react any suitable ketone on hand (acetone, methyl ethyl ketone..).
I'm interested in references. I'll also search some databases soon.. right after my current course ends 
|
|
|
Kinetic
Harmless

Posts: 45
Registered: 31-12-2004
Member Is Offline
Mood: fine
|
|
Nitrosation references
I'm certain that the general procedure for propiophenone can be applied to other ketones. As the reaction is acid catalysed, any ketone which can
react on either side will prefer to nitrosate on the most heavily substituted side. Thus the nitrosation of methyl ethyl ketone will give
3-oximino-2-butanone (aka butane-2,3-dione monooxime). Since the product is conjugated it should be less nucleophilic than the starting ketone, and
only one side will be nitrosated. I'm not sure if its possible to nitrosate both sides of a ketone, even if they are not blocked by alkyl or aryl
groups. Beilstein gave no hits for the 'double nitrosation' of acetone.
I suspect the NaCl in the patent is acting as a drying agent. Usually this reaction is performed using an ester of nitrous acid, and concentrated HCl
catalyst. The authors of the patent probably keep the conditons as dry as possible to favour the formation of the isopropyl nitrite in-situ. However,
there are reactions which can do away with nitrite esters altogether. The best reference I have for this I posted at the Hive a while ago: Synlett, 2004, 2315-2318, which you can access if you sign up for a free trial.
Most other references use nitrite esters, such as the synthesis of dimethylglyoxime from Organic Syntheses. Nitrosyl chloride has also been used to effect the transformation, but this appears to give
undesirable byproducts and so is not in general use.
Nitrite esters are easy to make from alcohol, acid and NaNO2, but the lower order ones are gases, making them a bit more tricky to work with. For the
nitrosation of MEK using alkyl nitrites, see Chem. Ber., 11, 323 (1878), Chem. Ber., 28, 1518
(1895) and Chem. Ber., 35, 3292 (1902).
|
|
|
|