BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Using an electric field to induce a dipole.
In my physics class they were discussing the effect of an electric field on molecules and just dipoles and such in general. The text book went into
some practial examples and said that when a non-polar molecule is put into an electric field it can induce a dipole in the molecule. Now, there were
no specific examples given, but could a non-polar solvent have its solvating properties altered significantly by introducing an electric field?
Like, for example, one could take a small amount of sodium chloride and add to hexane then subject the mixture to an electric field and magically the
salt would dissolve, then the field could be discontinued and the salt would precipitate? Excuse me if this seems impossible, physics and such are
not my relm of expertise. But it did seem interesting to think of and my physics teacher was no help (has this been discussed elsewhere?).
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I don't think so? I know the induced dipole stores energy sort of like an atomic spring (hence greater than unity dielectric constants of most
insulators).
Worth a try, but I bet it'll have to be near breakdown voltage (which means several kilovolts across a rather small cell), and even if you did
get the salt to dissolve and ionize, it would electrolyze and suck massive current from the power supply. 
Tim
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
This is how an O2 molecule is pulled apart in a strong electric field, and the two parts then can combine with another O2 molecule each to form Ozone.
Whenever an insulator breaks down, it is initially due to electrons being pulled out of their normal, stable position, by being pulled apart by the
strong field. The arcing would come later, as the avalanche of electrons knocked more loose. When the electric field, in volts/meter approaches or
exceeds the strength of the electron's molecular bond, in it's small scale, the bond is first strained (the atomic spring you mentioned)
then broken.
|
|
|
Madandcrazy
Hazard to Others
  
Posts: 117
Registered: 11-5-2005
Member Is Offline
Mood: annoyed
|
|
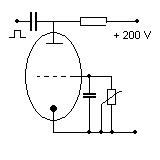
The atomic spring of a electron  can be can be
proved with a electron tube, for instance with a triode.
The control grid of the triode causing a voltage impulse on the catode of the electron tube.
|
|
|