treelike
Harmless

Posts: 7
Registered: 6-2-2006
Location: Scotland
Member Is Offline
Mood: No Mood
|
|
Phenol from Paractemol (Acetominophen)
I could do with a tiny bit of Phenol for studying colour changes with mushrooms. Phenol is one of those chemicals (others include FeSO4 and NH3) which
produce a colour change when applied to the flesh of certain mushrooms and this can be invaluable in identifying different species which otherwise
look the same.
Obviously I only need tiny amounts of Phenol to apply to the mushroom flesh so I was wondering if it could be made from the common (and extremely
inexpensive) painkiller Paracetamol? Looking at the formula on this page:
http://en.wikipedia.org/wiki/Paracetamol
all you would need to do is "break off" the amino bit but then maybe I'm being a bit simplistic.
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
There is tread about possibility of making phenol from paracetamol. http://www.sciencemadness.org/talk/viewthread.php?tid=5617
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Better done from aspirin, the amino group on doesn't fly off that easily, except with nitrous acid and then you have to work to make sure the NH2 gets
replaced with H.
Aromatic carboxylic acids decaboxalate, the more HO- substitutions the easier; gallic acid does so by boiling a solution of it. The thread chromium
posted ends up the phenol topic suggesting the decarbox route, before it drifts off into boom making.
If you're feeling experimental, copper ions help some decarboxlations, you might try runs using the cupric salt of salicylic acid, do a run with
copper oxide mixed in with sodium salicylicate, and so on.
Vogal has a prep for p-hydroxybenzoinc acid that starts with salicylic acid and cooks off phenol ( 2 Ksalicylicate => phenol + para KO-bzCO2K )
|
|
|
treelike
Harmless

Posts: 7
Registered: 6-2-2006
Location: Scotland
Member Is Offline
Mood: No Mood
|
|
Ah so I should be able to mix some aspirin with NaOH solution, boil off the water and get some phenol distilling over eventually. I'll give it a
try.....
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
If you want to try, you should first dissolve the aspirin in boiling water, filter to get rid of most of the other stuff used to make the tablets, and
cool to let the acetal salicylic acid,/ salicylic acid, to crystallize out. And use uncoated aspirin.
|
|
|
Maja
Hazard to Others
  
Posts: 143
Registered: 27-2-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by not_important
If you want to try, you should first dissolve the aspirin in boiling water, filter to get rid of most of the other stuff used to make the tablets, and
cool to let the acetal salicylic acid,/ salicylic acid, to crystallize out. And use uncoated aspirin. |
Don't take his suggestion... Water doesn't work for that purpose... Use Acetone or IPA. ASA is soluble in Acetone while other impurities aren't..
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Actually it does work, for treelike's purposes. They're just going to dump it in aq lye anyhow. Boiling water hydrolyzes it to acetic and salicylic
acids, the acetic will partially boil off. 15 ml of boiling water dissolves 1 gr of salicylic acid while it takes something like 450 ml of cold water
- check your Merck Index. The junk in the tabs he'll want to get rid of is mostly insoluable in water, microcystalline wax _will_ dissolve in acetone.
Worked for me when de Gaulle was in charge, still worked when Boy Wonder took over in the U.S.
|
|
|
treelike
Harmless

Posts: 7
Registered: 6-2-2006
Location: Scotland
Member Is Offline
Mood: No Mood
|
|
Wow! Thanks for the suggestions. I'll try the water route first as I'm running extremely low on IPA. A bit of colourless or white impurity won't
probably be a big problem anyway. Will post a follow up when I get round to doing the experiment.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Note that you are heating something that is alkaline to a fairly high temperature, like the benzoic acid decarboxilation this can be hard on glassware
- although not quite as hard as less heat is needed. If you are doing this a lot, or in large batches, making a retort from pipe might be better. For
just trying it, or getting aa gram or so of phenol, a run in a test tube is OK.
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
| Quote: | Originally posted by treelike
Ah so I should be able to mix some aspirin with NaOH solution, boil off the water and get some phenol distilling over eventually. I'll give it a
try..... |
Won't you get the sodium salt of phenol after the initial heating and then need to react with an acid to get the sodium salt of the acid plus phenol?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
No. The process from Vogel, using KOH, is
2 Ksalicylicate => phenol + para KO-bzCO2K
You're starting with the salt of the carboxylic acid, the salicylate decaboxylates shedding it's alkali onto another salicylate's phenolic HO- The
reaction goes both ways, as this is not being done as a sealed reaction phenol exscapes.
If you use the potassium salts you also get mostly para-hydroxy, sodiumshould give mostly ortho-hydroxy wwhich looks like no change. The hydroxy-acid
can be recycled, either by treating it with water+CO2 to free up the phenolic group or by mixing it with the proper amount of salicylic acid to do the
same - get the phenolic HO- as HO instead of K/NaO-.
It's only a fair to poor yield, but aspirin is cheap in Europe and NA.
|
|
|
Maja
Hazard to Others
  
Posts: 143
Registered: 27-2-2006
Member Is Offline
Mood: No Mood
|
|
Inactive ingredients in some aspirin tablets.... :
Black iron oxide
Brown iron oxide
Carnauba wax
Corn starch
D&C yellow #10 aluminum lake
FD&C yellow #6 aluminum lake
Hypromellose
Methacrylic acid copolymer
Polysorbate 80
Powdered cellulose
Propylene glycol
Shellac
Sodium lauryl sulfate
Titanium dioxide
Triacetin
May contain talc
Triethyl citrate
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Generic non-coated, non-buffered
Active Ingredients
Per Tablet: Aspirin 325 mg
Inactive Ingredients
Dicalcium Phosphate Dihydrate; Glyceryl Triacetate; Hypromellose; Starch; Talc
In the brand you listed the yellow dyes colour the coating, the iron oxides are in the printing on that. The TiO2 and shellac, and maybe methacrylic
polymer, are likely part of the coating.
Cellulose and starch are for bulk and to help set the disintegration properties of the tablet.
When you're using a medicine as a raw material source, try to get generic, uncoated or as simple of a coat as you can find, without printing on the
tablet. Generics are cheaper in part because they tend not to have extras in them.
|
|
|
treelike
Harmless

Posts: 7
Registered: 6-2-2006
Location: Scotland
Member Is Offline
Mood: No Mood
|
|
OK, so I got aspirin and started some experimentation today. I don't have a lot of equipment here and could do with some micro-scales so bear with me.
First I needed some way to prove I had made phenol and found out in one of my old chem books that ferric chloride makes a violet colour with phenol. I
mixed some ferrous sulphate, from an old chemistry set, with sodium hydroxide and warmed and shook the solution to make a nice brown precipitate of
"ferric hydroxide". Then I added a bit of hydrochloric acid to get a cloudy yellow ferric chloride solution (don't ask me why it was cloudy).
Next I tried hydrolysis of aspirin. I put two aspirin tablets with some water in a small test tube with a pellet of sodium hydroxide and heated it.
Almost immediately there was a yellow colour around the pellet of NaOH which had fallen to the bottom of the tube.

After some more heating, the solution got mixed up and the yellow colour faded so I guessed it needed a bit more NaOH. After adding another pellet and
warming the whole liquid turned dark yellow, almost orange.

I'm not sure where this yellow colour is coming from. The aspirin added ingredients were listed as potato starch, lactose and talc. As far as I know
none of them will produce anything yellow with NaOH so it must be from the acetylsalicylic acid itself. Could it be some decomposition product of
phenol??
Shortly after this, the solution decided to suddenly spurt over my finger so I swiftly made my way to the sink to wash my hands, hot caustic alkali
generally not being a desirable thing to have on your skin. There was no damage done. That'll teach me not to wear gloves.
Fortunately there was enough liquid left in the tube to try it with the Fe2(SO4)3. I got some various interesting colours starting with wine red as
shown here:
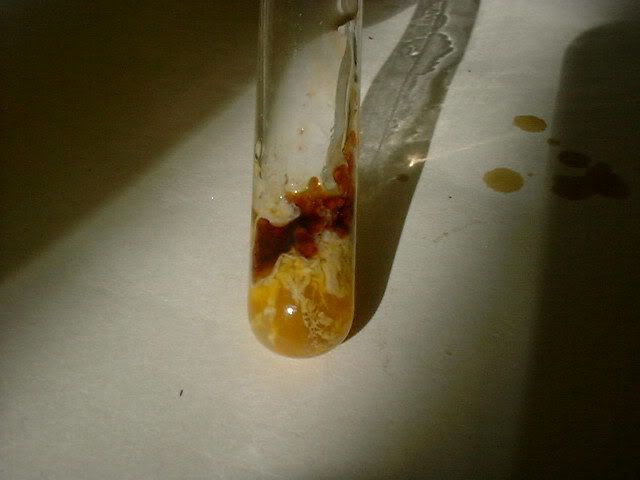
On washing out the tubes after the expt I could see various other colours including violet and blue. This made me think that I had indeed made phenol-
until I read one of the threads above which indicates that salicyclic acid can also make a purple colour with Fe3+
I never really noticed a phenolic smell (not sure if I would even recognise one) but then it occured to me that I should have added a bit of
hydrochloric acid because any phenol made was probably locked away as sodium phenoxide due to excess alkali. HCl may have freed up the phenol and made
it smell.
I guess the biggest question this experiment has produced for me is what the yellow colour was when the aspirin first reacted with the NaOH.
I think I will re-attempt on a slightly larger scale but perhaps boil away most of the liquid, acidify and hope that a phenol or phenol aqueous
solution can be distilled over eventually. Also I'll get a purer ferric solution to test it out.
[Edited on 2007-8-24 by treelike]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The FeCl3 is a more or less generic one for phenols as a family.
Heating complex organics (starch, lactose) with NaOH and air will give you coloured oxidation and condensation product ( 'gorp' ). Plus there are
several minor ingredients in even generic plain aspirin, was that an exhaustive list you gave?
The phenol will actually distill out of the dry mix. On this small of a scale, if you incline the tube so the upper section stays cool, maybe wrap a
damp cloth around the top, the phenol will condense there.
Check that reference in Vogal, which can be downloaded. You start with the salt of the acid, with the phenolic group free. There needs to be (roughly)
the right amount of NaOH to leave half the total Ar-OH free, after some of the NaOH is tied up as carbonate from the CO2 popping off salicyclic acid
NaOH + Ar(OH)(CO2Na) => Na2CO3 + Ar(OH)(H)
BTW - I'd start with a few cc water and the aspirin, no hydroxide. Boil it gentle for a few minutes, you should smell acetic acid. Keep boiling it,
adding water as needed, until that smell decreases. Then add the NaOH, and carefully boil it dry, then heat strongly. That way you're not tying much
NaOH up with acetic acid, and you'll have a better mix of salicyclate and NaOH.
[edit] I _cannot_ type at all.
[Edited on 5-8-2006 by not_important]
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
Smell of phenol is very strong and quite different. Some printed circuit boards have slight phenolic smell and some types of watercolors and gouaches
in my childhood smelled like phenol. Unfortunately i do not know about modern types.
I converted aspirin to salicylic acid this way: powdered two 500mg tablets of aspirin and added 20ml acetone. This was stirred for some time and
filtered. After filtering i got almost (but not completely) clear colorless liquid.
This liquid was evaporated to dryness and NaOH solution was added. (NaOH soln was made beforehand by mixing 10ml distilled water and 1.8g NaOH)
Everything dissolved. I heated solution to boiling and then left to cool.
To this liquid i added aproximatele 11ml of 20% HCl. White precipitate immediately formed. This was filtered and dryed. I suppose that resulting white
powder was salicylic acid.
I did not prepare phenol from salicylic acid that time although this should be quite easy. One day i mixed very small amount of this salicylic acid
with some lime ( Ca(OH)2 ) and heated it strongly in test tube. I did not collect resulting phenol but some of it surely formed as there was very
clear smell of phenol.
[Edited on 4-8-2006 by chromium]
|
|
|
treelike
Harmless

Posts: 7
Registered: 6-2-2006
Location: Scotland
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Originally posted by not_importantHeating complex organics (starch, lactose) with NaOH and air will give you coloured oxidation and
condensation product ( 'gorp' ). Plus there are several minor ingredients in even generic plain aspirin, was that an exhaustive list you gave?
|
That's what I got from the box, whether the list *they* gave was exhaustive I cannot tell  Maybe for interest's sake I could try NaOH with starch or lactose one day and see if it goes yellow. Maybe for interest's sake I could try NaOH with starch or lactose one day and see if it goes yellow.
Thanks again for the practical suggestions- you guys are really helpful! I'm pretty sure I will get there now. Once I find some mushrooms to test the
phenol on I can make a little web page showing colour changes and post it to the general forum.
|
|
|
jimmyboy
Hazard to Others
  
Posts: 235
Registered: 1-3-2004
Location: Texas
Member Is Offline
Mood: No Mood
|
|
wouldnt it just be better to put aspirin in sulfuric acid to separate the acetic acid/salicylic -- then boil off the acetic and basify the remains
with potassium hydroxide or potassium carbonate - you would end up with potassium sulfate/potassium salicylate - heat them and phenol would form
just wondering
|
|
|
jimmyboy
Hazard to Others
  
Posts: 235
Registered: 1-3-2004
Location: Texas
Member Is Offline
Mood: No Mood
|
|
nevermind found my info in this thread
http://www.sciencemadness.org/talk/viewthread.php?tid=6363
[Edited on 17-8-2006 by jimmyboy]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
All he was after is the phenol, so direct base hydrolysis is the shortest, easiest route; it uses fewer reagents. The sodium acetate goes off as
methane, so it's not a problem.
|
|
|