carlituz
Harmless

Posts: 4
Registered: 27-10-2015
Member Is Offline
Mood: No Mood
|
|
Cyclopentadiene from alpha-pinene?
Hi everybody,
I was wondering if it's possible to synthesize cyclopentadiene starting from alpha-pinene commonly found in turpentine oil.
A hypothesis was an isomerization of alpha-pinene to camphene. Camphene should then pass a Retro Diels Alder reaction to give cyclopentadiene and
isoprene. Do you think it's possible? And in which conditions (acid, base, catalyst needed)?
Thank you
Carlo
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Catalytic conversion of alpha-pinene to camphene is definitely possible. But retro Diels Alder to get cyclopentadiene? No idea.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Camphene, when written 2,2-dimethyl-3-methylene-bicyclo[2.2.1]heptane, has a single bond C5-C6 where a double bond would be required for the desired
retro-Diels Alder fragmentation. The 3-methylene double bond cannot migrate to C2-C3 because of the 2,2-dimethyl substituent and cannot migrate to
C3-C4 due to Bredt's rule. Hence a retro-Diels Alder reaction of camphene is unlikely, though a retro-Diels Alder reaction of 5,6-didehydrocamphene
might be possible.
I thought of a route to cyclopentadiene as follows:
diethyl ketone + Br2 + HBr >> 2,4-dibromopentan-3-one + Na2CO3 >> divinyl ketone
http://www.orgsyn.org/demo.aspx?prep=cv6p0520
or
H2CO + NEt2H + Me2CO + hydroquinone (polymerization inhibitor) >> di(diethylaminoethyl)ketone dihydrochloride + heat >> divinyl ketone
http://chemistry.mdma.ch/hiveboard/newbee/000480940.html
paracetamol might be substituted for the hydroquinone (similar properties)
piperidine or pyrrolidine or dimethylamine (stinky) or proline esters, n-methamphetamine etc might be substituted for the diethylamine
divinyl ketone + H2SO4 >> cyclopentenone (Nazarov cyclization)
cyclopentenone + Al(OiPr)3 >> cyclopentenol (Horner-Wadsworth-Emmons reduction)
cyclopentenol + H2SO4 >> cyclopentadiene (simple elimination)
[Edited on 28-10-2015 by clearly_not_atara]
|
|
|
carlituz
Harmless

Posts: 4
Registered: 27-10-2015
Member Is Offline
Mood: No Mood
|
|
Thank you very much clearly_not_atara.
My challenge was to synthesize cyclopentadiene starting from alpha-pinene: the route via camphene was an hypothesis. Do you think that having
turpentine oil as a matter to begin with, it would be possible to get to cyclopentadiene? (maybe passing through dehydrocamphene?)
[Edited on 28-10-2015 by carlituz]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Oxidative degradation of camphene might proceed by KMnO4 cleavage of the 3-methylene to a 3-oxo followed by Baeyer-Villiger oxidation in which the
2-carbon migrates.
Cleavage of the ester gives a substituted cyclopentane. With H2SO4 you'll get if you're lucky a 3-isopropylidenecyclopentane-1-carboxylic acid.
The silver salt can be formed by adding AgNO3 to an aqueous sol'n of the sodium salt and collecting the precipitate. It can dissolve in
dichloromethane and react with NBS or similar to give 3-isopropylidene-1-bromocyclopentane.
Elimination and rearrangement with eg fused NaOH should end in sodium isopropylcyclopentadienide.
I'm not sure all of this would work, so keep looking, but good luck in any case.
[Edited on 28-10-2015 by clearly_not_atara]
|
|
|
Nicodem
|
Thread Moved
29-10-2015 at 01:20 |
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
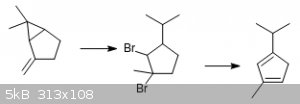
It's not pinene, but I found a much simpler synthesis of [a] cyclopentadiene in only two steps from a natural product, sabinene, which can be
steam-distilled from a common plant, Juniperus sabina:
http://en.wikipedia.org/wiki/Juniperus_sabina
http://en.wikipedia.org/wiki/Sabinene
A report in 1912 (second paper) reported varying yields of sabinene up to 16% from (possibly fraudulent) commercial savin oil, while a later report
claimed a 30% yield of sabinene from freshly distilled savin oil. Savin oil is steam distilled from twigs of savine juniper and sabinene is then
obtained as a fraction boiling at 162-166 C.
Sabinene can also be obtained from carrot seed oil, of which it comprises approximately 15%. Carrot seed oil may be commercially available.
http://grasasyaceites.revistas.csic.es/index.php/grasasyacei...
To make cyclopentadiene, sabinene is treated with anhydrous HBr in acetic acid or a similar solvent to doubly hydrobrominate the compound with
cleavage of the cyclopropane. The resulting 1-isopropyl-3-methyl-2,3-dibromocyclopentane is dehydrohalogenated to 2-isopropyl-4-methylcyclopentadiene
by treatment with base and possibly further deprotonated to the cyclopentadienide at the same time, facilitating separation of the product.
Not bad if you like hiking...
Attachment: v63-421.pdf (287kB)
This file has been downloaded 271 times
Attachment: agnew1912.pdf (246kB)
This file has been downloaded 224 times
[Edited on 31-5-2017 by clearly_not_atara]
|
|
|