RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Creating vacuum in sealed vessel via condensation of vapors
A while ago there were some threads discussing novel ways to create a vacuum without a mechanical pump and one way was to heat a vessel (like an old
propane tank) filled with air so that the hot air can escape - then close the vent on the tank and allow the tank to cool. This should reduce the
pressure in the tank to below ambient pressures.
Someone mentioned that a better way to create a vacuum, or at least a significant drop in pressure, was to place some water in the vessel and heat to
boiling - close vent - and allow to cool. The vapor -> condensation transition allows for much greater pressure drop than heating just air to the
same point (or even much higher temp).
I'm trying to find out how much water or steam is needed per cubic ft and how much of a pressure difference there will be once the water vapor has
condensed back to liquid.
Also, would this work with something like an alcohol or other more volitile liquid? I'm thinking that the energy needed to change from liquid to
vapor (and back) may work better with a liquid that doesn't require 540cal/g like water - but would an alcohol (or other liquid) create the same
pressure difference?
What would be some other liquids which would be good for this type of application? (note, this is theoretical, I'm not planning on heating a vessel
for this - simply research on compounds)
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
If you boil water in a vessel to fill it with steam, and then seal and cool it, the internal pressure will be the vapor pressure of water at the
temperature to which it is cooled. For a table, see: https://en.wikipedia.org/wiki/Vapor_pressure_of_water
A more volatile liquid, such as alcohol, will produce a weaker vacuum (and also, alcohol will cause flammability issues).
[Edited on 12-11-2016 by Metacelsus]
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
We covered this in another thread, lots of risks, poor resualts.
water in its liquid form and steam is 1:1700 so a small bit will do a large volume, but in the rarefied atmosphere you will only get to a vacuum
relivent to its vapor pressure at what the ambient temp is. So to get any usable vacuum for most would require chilling to make it deeper
Gravity pump, fill large bucked with water, put a lid on with 2 hoses, longer hose better vacuum hose lets water out another sucks air in. Better
sustained vacuum with out the risks of steam explosion!
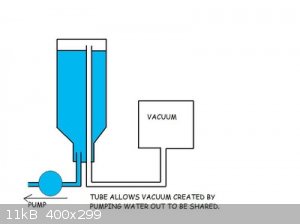
What are you trying to do/achieve by using this odd ball method?
As for fluids, silicon oil any thing with a high vapor pressure (Tends to need higher temp to boil)
[Edited on 11-12-2016 by XeonTheMGPony]
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by XeonTheMGPony  | We covered this in another thread, lots of risks, poor resualts.
water in its liquid form and steam is 1:1700 so a small bit will do a large volume, but in the rarefied atmosphere you will only get to a vacuum
relivent to its vapor pressure at what the ambient temp is. So to get any usable vacuum for most would require chilling to make it deeper
Gravity pump, fill large bucked with water, put a lid on with 2 hoses, longer hose better vacuum hose lets water out another sucks air in. Better
sustained vacuum with out the risks of steam explosion!
What are you trying to do/achieve by using this odd ball method?
As for fluids, silicon oil any thing with a high vapor pressure (Tends to need higher temp to boil)
[Edited on 11-12-2016 by XeonTheMGPony] |
Thank you for the reply! What you stated in the first paragraph is exactly what I was looking for. I'm not looking at creating/using vacuum as
applied to chemistry but how it may be used in conjunction with sterling engine type application (again, not exactly the standard sterling engine) -
where vacuum can assist movement on either a cold or hot end of a "piston".
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
look up : Organic Rankin cycle engine.
|
|
|