Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Oxidation of manganese-EDTA with persulphate
Tonight I attempted to produce NaMnEDTA (EDTA = ethylenediaminetatraacetate).
An earlier experiment showed that when Na2EDTA is dissolved in water and the stoichiomethic amount of MnSO4 is added, this will not yield a solution.
The presence of SO4(2-) lowers the pH so much that EDTA (and its presumed Mn2+ complex) will no longer remain in solution.
Therefore, in tonight's experiment, I added 2 parts of NaHCO3 to 1 part of Na2EDTA in water and boiled it shortly to drive off most of the carbonate.
The pH was around 9. Then I added 1 part of MnSO4, which dissolved under further release of CO2.
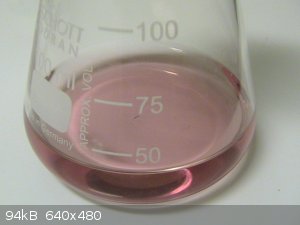
This solution was divided into two equal parts by volume. To one part of the solution 1/2 part of Na2S2O8 was added, so now Na4EDTA, MnSO4, and
Na2S2O8 were present in 2:2:1 ratio. The solution remained colourless, but on heating, the colour changed to pink around 80C. When heating to boiling,
a sweetish scent was observed.
My question is: what is the scent is caused by?
If found this article, which deals with the oxidation of NaFeEDTA, forming the ethylenediaminetriaceticacid from the tetraacetate
in absence of oxygen (absence of O2 is not likely in my setup). Maybe Mn(3+) is capable of similar reactions. But stil I wonder what may have formed
that caused the sweetish scent.
Since I didn't notice the scent when NaEDTA, NaHCO3 and MnSO4 were boiled before the persulphate was added, it seems obvious that some sort of
oxidation reaction is going on.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Sweet smells are generally associated with esters, though not exclusively. Still, esters tend to smell strongly, so even very small amounts are
detectable, and they're frequently produced by accidental oxidation. So if you're trying to guess a category for this compound, esters would probably
be a good first place to start. When you're heating a solution of transition metal ions and organic compounds in the presence of oxygen, there's a
very good chance that there's some catalytic oxidation of one of your organic compounds happening. Since you already have a bunch of acetate groups,
there's a very good chance you're dealing with an acetate ester, possibly methyl acetate or hydroxyethyl acetate.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Okay, thanks, Melgar. I just repeated the experiment, and the same smell appeared. It's also reminescent of ethanol. somehow. I have the impression
that the reaction is thermally driven, so I'll retry by adding the persulphate to a cold solution.
|
|
|
|