Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
Hydrogen Peroxide from Ozone?
Hey, I am new to this Forum, and I'm interested in the Formation of H2O2 from Ozone...
I've read that someone in this Forum done this before, but I could find, Who and How..
Are there some others that have tried this?
I probably will test some ways when I buy a cheap eBay China O3 Generator
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Ozone generators kind of suck for synthesis unless you have a pure O2 line for them. Otherwise, you have to run so much air through your reactor that
it blows away your product as it forms, and your solvent too. Have you looked into what it'd take to make it via barium peroxide or similar?
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
I would have a supply of O2 but ok.
I think The Production of Bariumperoxide isn't that hard, I ordered a Quartz tube recently and it should arrive soon.
You just should Heat Bariumoxide (from Bariumsulphate) under a Steady Stream (maybe the Balloon technique as in hydrogenation would just Work fine)
of oxygen rich air... (works probably fine With normal air) and you get some peroxide which is then reacted With sulfiric acid to Form Hydrogen
Peroxide and Bariumsulphate Which will Settle down
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
More or less. If I'm not mistaken, you heat the BaSO4 with carbon or sulfur to get barium sulfide, which reacts with water to yield H2S and BaO.
Then just heat BaO in oxygen or air again to regenerate the peroxide.
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Melgar  | | More or less. If I'm not mistaken, you heat the BaSO4 with carbon or sulfur to get barium sulfide, which reacts with water to yield H2S and BaO.
Then just heat BaO in oxygen or air again to regenerate the peroxide. |
I looked at this process and was unable to determine the temp needed but Wiki states that "high temps" are needed. From looking at other barium
compounds, this could be pretty high as BaSO4 decomposes near 3,000F but the addition of C should lower that temp I would think.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I don't think that you would even want to use barium sulfide if you could make it. And if you do make BaS you'll very likely end up with tiny amounts,
rendering the process not much more efficient than an ozone generator.
However, I think you could use concentrated hydrobromic acid to reduce barium sulfate to the sulfite and subsequently hydrolyze this to the bromide
with loss of SO2. This releases SO2 rather than H2S which is thousands of times less toxic. The product BaBr2 can then be converted to BaO using
concentrated NaOH (BaO precipitates).
Bromine is also produced by this rxn but it too is less dangerous than H2S if you ask me. Barium bromate is insoluble in water so it is possible that
by treating the product BaBr2/bromine water with NaOH the oxidized bromine can be efficiently converted to barium bromate, filtered, and the barium
oxide then precipitated by reducing the temperature and concentrating the solution. The bromine may then be recovered as NaBr.
If you're lucky you can convert BaSO4 to a mixture of BaBr2O6 and BaO while recovering nearly all of the Br as sodium bromide. I haven't tried this
however. Not sure what you can do with barium bromate but it's probably good for something; sulfuric acid converts it to a very dangerous explosive.
[Edited on 5-6-2017 by clearly_not_atara]
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
Wikipedia States the Process is like shown in the picture below
So there is no Sulfide forming, and the Barium oxide is obtained..
Yes, Carbon monoxide is forming, but I-m Making it in the outside With ventilation
Wikipedia says that there is another Process With the Carbonate an Carbon to Form the oxide, at around 1030°C/1890°F, but the Temp should be a
little Bit lower, when the oxide is used..
Barium Nitrate decomposes to Oxide when heated.. I could May get it from sparklers... But thats probably inefficent.
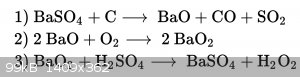
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Wikipedia quite clearly states the reaction is as follows:
BaSO4 + 2C>> BaS + 2CO2
http://en.wikipedia.org/wiki/Barium#Occurrence_and_productio...
Wikipedia does say that the carbothermal reduction of barium *carbonate* can be used to make barium oxide like so:
BaCO3 + C >> BaO + 2CO
However I do not see the reaction you claim on Wikipedia, and it looks wrong to me. In particular the reaction 2 CO + SO2 >> 2CO2 + [1/8 S8 or
1/2 S2] should be favorable at every temperature so CO and SO2 should not appear as products of the same reaction.
EDIT: this is interesting:
| Quote: | | Previously, hydrogen peroxide was prepared industrially by hydrolysis of the ammonium peroxydisulfate, which was itself obtained by the electrolysis
of a solution of ammonium bisulfate |
[Edited on 6-6-2017 by clearly_not_atara]
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
I have the reaction from another Wikipedia site.. not from the English One, which is probably für Best one..
I've seen that One With the electrolysys before but I couldn't Really find actual Proves or similar... and ammoniumbisulfate is quite expensive and
hard to get
[Edited on 6-6-2017 by Amoled]
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
| Quote: |
ammoniumbisulfate is quite expensive and hard to get
|
@Amoled you can make your own ammonium bisulfate by slowly adding a mole of ammonia(aq) to a mole of sulfuric acid.
If you add too much ammonia then you can heat ammonium sulfate up to about 250*C, I make the assumption of 250*C because according to the wiki
ammonium sulfate decomposes before its melting point.
(NH4)2SO4 → (NH4)HSO4 + NH3
https://en.wikipedia.org/wiki/Ammonium_sulfate
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
The Electrolysis should form Ammonium persulfate, which then reacts with Water to the corresponding peroxide and Ammonoum sulfate
In warm Water it decomposes Into Ozone or Radical sulphatr ions
I cant find any direct Information on the forming of Peroxide, maybe the Ozone reacts With the Water to Form H2O2
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 184
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
Barium nitrate costs about $5.50 per pound on hobbychemicalsupply.com, but their website is down temporarily. I have placed an order for metal powder
with them before, and it arrived on time and the product was good. Here is a link to barium nitrate from an availible supplier, but I cannot attest
for them, as I have never ordered from them.
Link: http://www.pyrochemsource.com/Barium-Nitrate-BARIUM-NITRATE....
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
I know of Barium nitrate, but in my Country, Nitrates are really hard to get, and the only Way I can get Nitrates is, from Sparklers the Barium
Nitrate, which is Way to expensive, Or Calcium and Ammonium nitrate from fertilizier
So maybe I could treat the Ammonium nitrate With Bariumhydroxide when it is possible...
Barium Nitrate is the easyiest Way for making Bariumoxide... But its hard to get here..
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
Bariumhydroxide decomposes at around 500-600°C to BaO maybe that would Work too... But thats probably hard Because in higher temps (700°C it goes
back to the Bariumhydroxide, but the Water should evaporate fast, so that the reaction goes to the of Bariumoxide formation)
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I'm not sure why everyone freaks out about hydrogen sulfide so often. The stuff can be smelled at extremely low concentrations, and as long as you
don't ignore the smell, and are somewhere ventilated, it'd be nearly impossible for any harm to come of exposure to it. Contrary to popular belief,
the "deadening" effect on your olfactory receptors is not a quick process and requires high concentrations. Consider that H2S is a signalling
molecule in your own body, so it's not like lead or mercury, where even tiny amounts are bad for you.
Barium sulfide is toxic, and in fact any barium salts except sulfate are toxic, but those dangers can be mitigated. I work with cyanide and other
substances of similar toxicity regularly. As long as you respect and understand them, they're actually not as scary as people tend to think they are.
Of course, working with them requires you to do exhaustive research into every aspect of the reactions you'll be doing, know all the dangers, all the
signs of toxicity, any treatments for toxicity so you can have them available, etc., but the risks are manageable.
[Edited on 6/6/17 by Melgar]
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
Me neither, but couldn't I just let the excess H2S, Stream through a NaOH Solution to form Na2S... and vent the rest Away?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The reaction sequence which claims that barium sulfate can be reduced directly to barium oxide was posted on the German Wikipedia eight years ago
with the only possible citation being "Winnacker Küchler, Chemische Technololgie Band 1, 3. Auflage 1970, Seite 514". I haven't tracked this down.
However, it's not very convincing.
It's my understanding that barium sulfate is *normally* converted to barium oxide by a multi-step process, which certainly wouldn't exist if you could
get there in one step:
BaSO4 + 2 C >> BaS + 2 CO2
BaS (aq) + CO2 (aq) >> BaCO3 (s) + H2S (g)
BaCO3 + C >> BaO + 2 CO
https://de.wikipedia.org/wiki/Bariumoxid#Darstellung
However, apparently this can be done with Na2CO3 instead so H2S release was never necessary (derp!):
BaS + Na2CO3 >> BaCO3 + Na2S
http://en.wikipedia.org/wiki/Barium_carbonate (scroll down)
| Quote: | | I'm not sure why everyone freaks out about hydrogen sulfide so often The stuff can be smelled at extremely low concentrations, and as long as you
don't ignore the smell, and are somewhere ventilated, it'd be nearly impossible for any harm to come of exposure to it. |
It can still render a room lethal, so it's worthy of caution. Also it doesn't look good for the legal status of amateur chemistry if we're found to be
venting H2S (or CO for that matter) to the atmosphere all the time. And it's hardly considerate to your neighbors if you're doing it outside of a
commercial laboratory which is clearly the situation here.
All told, it's certainly not my first choice. I had assumed that just about anyone would like H2O2 so I wanted to post an accessible route.
Speaking of which, how do you trap carbon monoxide? I've never known.
| Quote: | | Barium sulfide is toxic, and in fact any barium salts except sulfate are toxic, but those dangers can be mitigated. |
All metal salts except Na/Ca/Mg/K/Fe/Zn are toxic, but at least they stay in the condensed phase.
=================================================================
EDIT: this is awesome:
http://www.peroxychem.com/media/90826/AOD_Brochure_Persulfat...
[Edited on 6-6-2017 by clearly_not_atara]
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
So its all end up at Bariumcarbonate, Reduced by Carbon to the oxide, the Carbonate is easy to get, so no need for the steps before..
Carbon Monoxide could maybe Trapped With Hemoclobin to Form a
Carboxyhemoglobin complex
The Hemoclobin could be brought at Chemical supply company
[Edited on 6-6-2017 by Amoled]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Barium isn't cheap though, and it's quite heavy. In fact, it got its name due to just how heavy it is. So having a way to regenerate it would be
good.
@not_atara H2S and CO are both flammable, and if you have enough that they'd be dangerous, they're typically burned if they aren't going to be used,
at least in industry on smaller scales. A modified torch head or a bunsen burner can work for this, as long as you have something like a hand torch
set on low that can reignite the gas stream if it stops temporarily. I was assuming OP wanted to produce somewhere on the order of 100 mL at a time,
which wouldn't be especially dangerous as long as it was outside or vented. H2S smells like sewer gas anyway, so neighbors would be more likely to
assume someone had a sewer line back up, not that someone was doing chemistry experiments nearby.
The easiest way to convert barium sulfate to the oxide would just be to heat it in a refractory retort with an oxyacetylene torch until it released
SO3. (for the sulfuric acid you'd need for the other step; fun times!) Then after it cooled down, immersing it in water and boiling it to dry, venting
the gas through a torch flame. Once it's dry, continue to heat while running a stream of air over it, I imagine?
But since OP seems to want to buy hemoglobin to trap the carbon monoxide, I'm guessing he's not an experienced chemist at a commercial laboratory, and
processes involving high temperatures often blow up if any water is trapped somewhere in the apparatus. So I'm hesitating to recommend anything like
that. Personally, I've never been able to make more than a few grams of anything with electrosynthesis, and what I have made has inevitably been
contaminated by whatever random crap happened to fall off the electrodes. It seems like an easy way to make a mess, without having any idea regarding
what you actually made, how to isolate it, or where things went wrong, assuming you messed up somewhere, which is very likely. More traditional
syntheses, you isolate the product after each step, and make sure that it was successful. But ultimately, I guess it's up to OP to decide.
I don't suppose it's possible to react oxalic acid in aqueous solution with barium peroxide to form the oxalate salt and hydrogen peroxide? Oxalate
salts are incredibly simple to convert to their carbonates, losing a molecule of CO in most cases.
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
Thanks,
Barium carbonate cost like 4,50€/kg so it's Not so bad,
I've suggested Hemoclobin, to not have another Flame, and its cheap, Cow Blood would be disgusting but also ok..., but I will look Into burning CO
I would samt to isolate each Product and then go further.
The Bariumperoxide would be Converted With diluted Sulfiric acid to the Sulfate, maybe it can be Converted to Bariumoxalate With Oxalic oxide...
___________________________________________________________________
I have some pure Sodium Percarbonate here, can I treat it With Lauric Acid to get unsoluble Sodium Laurate, or will the Acid react With the Peroxide?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Sodium laurate forms a lyotropic phase. If this is mixed with peroxide the result will be a large expanding foam ("elephant toothpaste") and possibly
a fire. I strongly recommend you do not attempt this.
It makes more sense honestly to dissolve BaO2 with aqueous (HCl (recalling that peroxide is stabilized by acid), titrate to
pH 4-5ish with a solution of Na2CO3, collect dilute peroxide by distillation, and then convert the remaining BaCl2 to the carbonate by adding water
followed by more Na2CO3 until precipitation ceases. HCl and Na2CO3 are pretty cheap. )
EDIT: wrong, only HF can do this, and don't use that
Alternatively the solids comprising BaCl2 and Ba2CO3 are dissolved in HCl and heated to release all carbon dioxide, then the solution is treated with
NaOH, concentrated and cooled to recover barium oxide. Industrial processes are not always the easiest method for home chemists.
[Edited on 8-6-2017 by clearly_not_atara]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by clearly_not_atara  | | It makes more sense honestly to dissolve BaO2 with aqueous HCl (recalling that peroxide is stabilized by acid), titrate to pH 4-5ish with a solution
of Na2CO3, collect dilute peroxide by distillation, and then convert the remaining BaCl2 to the carbonate by adding water followed by more Na2CO3
until precipitation ceases. HCl and Na2CO3 are pretty cheap. |
Actually, that makes no sense at all. H2O2 is stabilized by OXOacids, and if you mixed hydrochloric acid with it, you'd get a nice safe cloud of
chlorine gas in your face.
At least when I give people dangerous advice, I get my facts straight first.
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
Also, With 31% HCl i would only get like 15-20% Peroxide Solution and had to distill the Peroxide off from the Good soluble Bariumchloride, and
Distilling H2O2 With Metal ions present isnt that what i want....
I could also buy 12% H2O2 instead and distill it....
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
My bad, I definitely should have at least guessed HCl would react with H2O2 and looked it up or something. Regardless it is Bronsted acidity and
specifically the absence of hydroperoxide anion which stabilizes H2O2 solutions which is why HF works.
I don't think it would be terrible to distill H2O2 from a solution containing barium salts because barium does not undergo redox cycling the way that
transition metals do. However because barium peroxide is insoluble the reaction may not be successful. In fact the whole idea of using a soluble
barium salt is probably not going to work.
Barium benzoate is insoluble which may be useful since benzoic acid can be distilled off; I'm not sure if this would also be true of oxalic acid, as
no boiling point is available.
[Edited on 8-6-2017 by clearly_not_atara]
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 184
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
Distilling hydrogen peroxide period is usually not the method used to concentrate it. If you buy 12% peroxide for example, evaporating on a hot plate
is generally the better method. If you have peroxide containing barium chloride it is probably best to precipitate it using a metal sulfate, one with
a less soluble chloride, then chill the solution to ice cold, and filter it. There will still be some chloride dissolved, and that would be a problem
for concentrating it. Trace quantities of certain ions will catalytically decompose the peroxide (permangante and rare earth oxides the fastest, but
plenty of things will) and you could lose most of your yield when distilling.
I would recommend concentrating the 12% peroxide, or for higher concentrations BaO2+H2SO4 and filtration.
|
|
|