biomechem
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Chiral centers, racemization of diastereoisomers, name of cpd with 3 chiral atoms.
Hello again,
1. Does the compounds with three chiral centers have special name analogously to diastereoisomers with two chiral centers?
2. I know that pure enantiomers may be racemized, so the question is is it possible to change configuration of diastereoisomers?
I think that when it comes to E/Z configuration it may be relatively easy, but what about policyclic compounds?
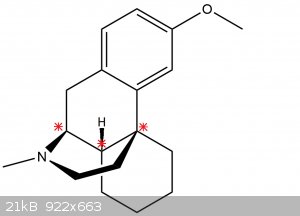
3. According to question number 2 is it possible to change configuration of the compound presented below?
I tried to figure out how many assymetric atoms does the compound have, and the asterisks are my types, am I right?
Finally would it be possible to change configuration of second chiral center to obtain L-derivative? Or a whole ring with nitrogen should be rotated?
And if the answer is yes then a steric hindrance is the problem in this case, is my deduction sensible?
BTW. Is it possible that by changing a middle chiral centre axial-equatorial configuration we obtain still CNS active substance? Would be rather
acting similar to levo or dextro version of compound presented?
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
A compound with three stereogenic centers simply has more diastereomers (stereoisomers which are not enantiomers).
Racemization is possible depending on the molecule. Certain functional groups facilititate racemization. Carbonyl for example, and any other EWG
really, allow racemization of the alpha position.
Now your molecule lacks such features and cannot be racemisized. Being a bridged decalin it is conformationally rigid and not all diastereomers might
be possible.
Edit:
After writing down several structures, I belive only 4 out of 8 diastereomers are possible. Numbering the stereogenic centers from nitrogen outwards
these would be the R,R,R and the R,S,R configuration along with the enantiomers of both compounds; the S,S,S (pictured) and S,R,S configuration
respectively.
The others are impossible because they are trans-bridged decalins, i.e. stereocenter 1 and 3 have to be cis for the molecule to be possible.
[Edited on 20-7-2017 by Sigmatropic]
|
|
|
|