amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
Gabriel-Colman rearrangement application
I am stuck in a synthesis problem and I have searched hard to find a clue but didn't find a solution
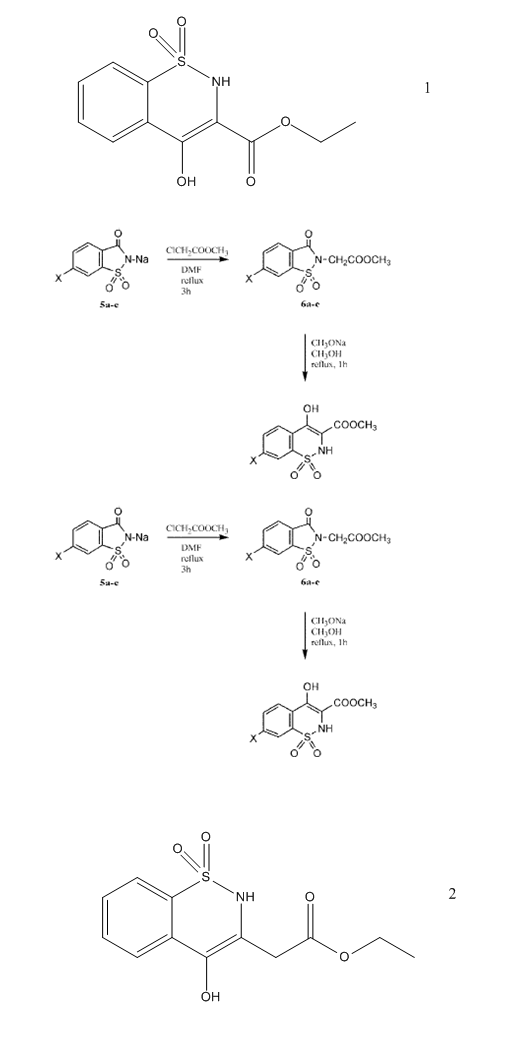
I want to get the compound number 2
The question is Can I apply Gabriel colman rearranngment to obtain compound no 2 the same way it was applied to get compound 1 . If not what shall I
do to get it?
amr h mahmoud
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Those heterocyclic azasulfones, with hexavalent S bonded to a secondary-amine H in the ring fused to a benzene ring, that you want to make, are
structurally very similar to the artificial sweetener Saccharin, except that in the latter the N and S are in a 5-membered ring, without the
side-chain, and with a keto-group in place of the -OH. It is about 3,500 times sweeter than sucrose, but it bears no structural relation to any
natural sugars.
Are you interested in those compounds as possible artificial sweeteners? Saccharin has been banned in Canada because of concerns over its long-term
safety, and possibly some other countries, but not in the USA. Sodium cyclamate, which is 300 times sweeter tham sucrose, also has a secondary-amine N
bonded to hexavalent sulfur (and to a cyclohexyl group), being also a sodium sulfonate salt, but it is not heterocyclic or aromatic.
[Edited on 12-3-08 by JohnWW]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The Gabriel-Colman ring expansion requires that the group attached to the nitrogen have an enolizable hydrogen :
N-CH2-CO2R
It might be possible that using mono-halo-succinic ester in place of the halo-acetic ester would give a precursor that could be hydrolysed to the free
acid, the decarboxylated to your target. This is just guesswork, not anything I've seen.
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
To not important ...........this is a good idea ......I will try
Thanks very much
amr h mahmoud
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
Some of my friends have doubt that decarboxylation will take place
Have anybody experience with that ? and what is the solution then?
amr h mahmoud
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
High boiling ether workup at the end? Thanks for the chemistry.
[Edited on 16-3-2008 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
Sorry chemrox ...plz give me an example how will that work and an evidence if u can
amr h mahmoud
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
There is an article which discuss the behavior of decarboxylation
3-Oxo-1,2-benzoisothiazoline-2-acetic Acid 1,1-Dioxide Derivatives. I. Reaction of Esters with Alkoxides
Celia B. Schapira, Isabel A. Perillo, Samuel Lamdan
Departamento de Quimica Organica, Facultad de Farmacia y Bioquimica, Universidad Nacional de Buenos Aires, Junin 956, Buenos Aires, Republica
Argentina
Reaction of 3-oxo-1,2-benzoisothiazoline-2-acetic acid alkyl esters 1,1-dioxide (1a-d) with alkaline
alkoxides was carried out under various conditions. Under mild conditions, o-(N-carboxymethylsulfamyl)benzoic acids dialkyl esters
(2a-d) were obtained with good yields. Reaction of 1a-d or 2a-d with sodium alkoxides under drastic conditions afforded
4-hydroxy-2H-1,2-benzothiazine-3-carboxylic acid alkyl esters 1,1-dioxide (3a-d).
Transesterification was observed when esters 1b-d were treated with sodium methoxide in methanol. Esters 3a-d were hydrolyzed in
concentrated aqueous sodium hydroxide affording the acid 6. Attempts to recrystallize 6 from water resulted in its decarboxylation to give
2H-1,2-benzothiazine-4-(3H)one 1,1-dioxide (7). Compound 6 could not be obtained by acid hydrolysis of esters
3a-d or by rearrangement of 3-oxo-1,2-benzoisothiazoline-2-acetic acid 1,1-dioxide (8). Different
experimental evidence supports the suggestion that rearrangement took place by ethanolysis of the carboxamide linkage affording the open sulfonamides
(fast step) followed by a Dieckmann cyclization (slow step). It was demonstrated that transesterification took place in the open sulfonamides 2
Plz if anyone has access to this article ,post it 
Journal of heterocyclic chemistry 17(6), 1281-8; 1980
[Edited on 28-3-2008 by amrhamed2]
amr h mahmoud
|
|
|
jizmaster
Harmless

Posts: 23
Registered: 1-2-2005
Member Is Offline
Mood: No Mood
|
|
beta-Keto esters are supposedly quite easy to decarboxylate by hydrolysis of the ester followed by acidification. It goes by a six-membered ring
transition state with the ketone removing the acid proton and formation of CO2 and the enol of the ketone, which tautomerises to the ketone.
The article you posted says it decarboxylated when trying to crystallise so it must be very easy for that compound, whatever it is!
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
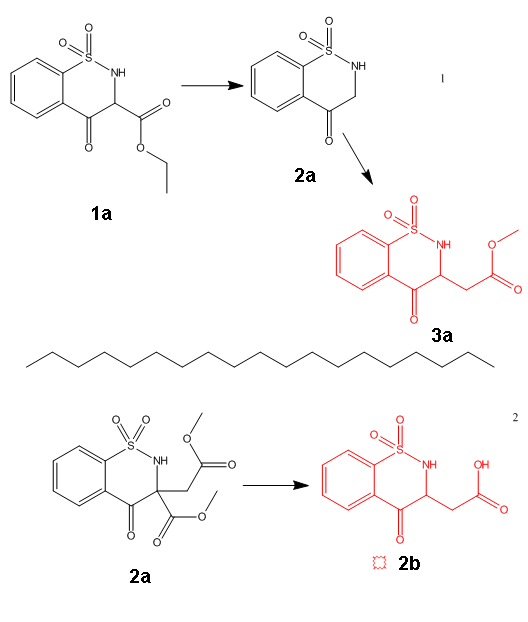
Plz revise this
In fig 1
To obtain the product 3a ,I can use decarboxylation then what to be done? .......protect amine and use methyl 2-bromoacetate ......Is there away to
obtain 3a without protection ???
In fig 2
Using 2-bromosuccinate with saccharin ....Will it give 1b which upon decarboxylation form 2b ???
amr h mahmoud
|
|
|