Bolt
Hazard to Others
  
Posts: 188
Registered: 26-3-2007
Member Is Offline
Mood: No Mood
|
|
Oxidative cleavage of alkenes using nitrobenzene
Most of my O. Chem books list only 3 or 4 methods by which to cleave a double bond. The most common are hot, acidic permanganate, ozone, periodic
acid, etc. In my search for different synthesis strategies involving double bonds, I remembered that I had read that vanillin has been prepared from
eugenol. On a lark, I searched at espacenet and came up with a few patents that all said nearly exactly the same thing.
If you search at espacenet for 'eugenol vanilllin nitrobenzene' in 'keywords in title and abstract' under advanced search, you will see many of these patents.
The bare bones of the process is as follows. Eugenol is first isomerized to iso-eugenol, and then the potassium isoeugenate is refluxed with
nitrobenzene (occasionally diluents are used), yielding vanillin.
In this example, the alkene is cloven at the double bond, resulting in an aldehyde. Does anyone know what the mechanism is? Also, is this is a very
specific process, i.e., will this type of cleavage only work on a few alkenes or is there potential for this method to work on a wide variety of
alkenes?
|
|
|
jizmaster
Harmless

Posts: 23
Registered: 1-2-2005
Member Is Offline
Mood: No Mood
|
|
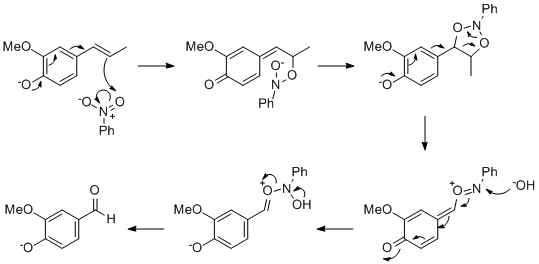
Interesting! I guess the mechanism might be something like this, maybe! I think it might need a phenol para to the propene to make the double bond
more nucleophilic, but maybe anything that makes the ring electron rich will do.
I found this but haven't had a good look at it yet:
http://www.sibran.ru/psb/phsb/papers/CSD2003_4_14e.pdf
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
It seems that the authors of SE82886 say that the reaction is general for many propenylbenzenes besides isoeugenol 
Attachment: SE82886C.pdf (119kB)
This file has been downloaded 1006 times
Chemistry is our Covalent Bond
|
|
|
jizmaster
Harmless

Posts: 23
Registered: 1-2-2005
Member Is Offline
Mood: No Mood
|
|
It does? I'll have to take your word for it! Do they say the nitrobenzene is reduced to aniline?
|
|
|
Bolt
Hazard to Others
  
Posts: 188
Registered: 26-3-2007
Member Is Offline
Mood: No Mood
|
|
According to the patents, azobenzene (Ph-N=N-Ph) is a biproduct. I'd assume that it is the major biproduct, as both wiki and orgsyn say that azobenzene is produced through the reduction of nitrobenzene. However, orgsyn also says that the product of the condensation of
aniline acetate with nitrobezene is azobenzene. Wiki also says that azobenzene may be produced from nitrosobenzene and aniline.... Who knows if the
azobenzene is produced directly from the reduction of nitrobenzene or some intermediate reaction?
[Edited on 26-3-2008 by Bolt]
[Edited on 26-3-2008 by Bolt]
|
|
|