| Pages:
1
2 |
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Endothermic freezing mixtures
This webpage states the following ratios of compounds yield a maximally achievable low temperatures:
NH4Cl............................30 g/100 g cold water..........................-5°C
NaNO3............................75 g/100 g cold water.........................-5
CaCl2·6H2O..................41 g/100 g ice......................................-9
KCl.................................30 g/100 g ice................................-10.5
Urea.......................... ..10 g/100 g ice....................................-10.8
NH4NO3....................14 g/100 g ice.....................................-13.6
NaNO3.......................15 g/100 g ice.....................................-13
NH4Cl........................25 g/100 g ice....................................-15.4
NH4NO3....................60 g/100 g cold water..........................-16.8
NaCl...........................33 g/100 g ice....................................-21.3
CaCl2·6 H2O................81 g/100 g ice....................................-21.5
NaBr............................66 g/100 g ice....................................-28
Ethanol.........................105 g/100 g ice .................................-30
CaCl2·6 H2O................123 g/100 g ice...................................-41
CaCl2·6 H2O................143 g/100 g ice..................................-55
KOH............................31 g/100 g ice....................................-63
H2SO4 (w = 66 %).........91 g/100 g ice...................................-90
MgCl2·6H2O................85 g/100 g ice....................................-94
Has anyone tried out colder ones like CaCl2.6 H2O, H2SO4, or MgCl2.6H2O?
I've tried CaCl2 (not the hexahydrate, anhydrous) about 143 g to 100 g cracked ice ratio and it didn't seem much colder than ice.
Without special equipment, what would be a good way to determine how cold those mixtures get? Maybe with the known boiling points of simple gases,
before (l) -> (g), e.g. CO2, B.p. -78°. HC#CH, B.p.: -84°. HCl, -85°. CH3.CH3, -88°. N2O, -88°. CH3.CH2.CH3, -42°. NH3, -35°, etc. try and
condense the gases.
If not then easier could be melting points of some common liquids before (s) -> (l).
[Edited on 2-5-2008 by Schockwave]
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
Hmmm, mixing CaCl2 with ice should result in an exothermic response due to the Heat of Hydration--this is why it is used in de-icing salts.
What you have here would seem to be a list of salts that decrease the freezing point of water to the specified temp. (Aside from the ones that arn't
salts, that is.)
[Edited on 5-2-2008 by ShadowWarrior4444]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by ShadowWarrior4444
Hmmm, mixing CaCl2 with ice should result in an exothermic response due to the Heat of Hydration--this is why it is used in de-icing salts.
|
Right; it warms up, I also noticed just adding the CaCl2 to regular water causes the solution to get very warm (heat of solution: -162 cal/g).
H2SO4 also heats up with H2O, but here they are saying it gets even much colder (with ice).
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
In my crude and small scale experiments using thermocouples, styrofoam cups, CaCl2 hexahydrate from big bag of Prestone ice melt and boiling water,
and snow, the lowest temp that I got was -38C IIRC. This was after cooling the snow and salt to -10C before mixing. Less cooling of the components
produced a corresponding result, it seems that there is just a certain number of degrees of cooling and that's that. So it all depends at what
temperature that you start out at. I tested all of the different ratios stated in the various literature and there was no difference in my hands.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Yes which is why it specifies CaCl2.6H2O. Ditto MgCl2, which I think is more hygroscopic.
Tim
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
What is the mechanism behind the cooling of water in this method? (What does the thermal energy go toward.)
Does the presence of a hygroscopic salt catalyze the melting of ice, thereby increasing the rate at which thermal energy is absorbed?
(Note: a slight bit of research indicates that this is true.)
Though some of the substances in the list call for cold water instead of ice--what is the mechanism behind this? Is energy required to dissosciate the
ions?
[Edited on 5-2-2008 by ShadowWarrior4444]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
The heat is used up by solvation energy. Solid ice acts as a "cold reservoir" and dissolves in the solution, cooling it. Apparently, some solubles
break up ice, absorbing what was crystalline binding energy. Likewise, some materials (urea, ammonium nitrate, etc.) become very cold on dissolving,
as the crystal structure is broken up.
I'm amazed KOH is on the list; NaOH becomes very hot when it dissolves! I can't imagine any big difference between the two salts that would make such
a diametrically opposed result.
Tim
|
|
|
ScienceGeek
Hazard to Others
  
Posts: 151
Registered: 22-1-2008
Location: Norway
Member Is Offline
|
|
Funny the reaction of Ammonium Nitrate and Barium Hydroxide Octahydrate isn't mentioned.
<object width="425" height="355"><param name="movie" value="http://www.youtube.com/v/GmiZ0huvZzs&hl=en"></param><param
name="wmode" value="transparent"></param><embed src="http://www.youtube.com/v/GmiZ0huvZzs&hl=en" type="application/x-shockwave-flash"
wmode="transparent" width="425" height="355"></embed></object>
Equation:
Ba(OH)2•8H2O (s) + 2NH4NO3 (s) → Ba(NO3)2 (aq) + 2NH3 (g) + 10H2O (l)
This better demonstrates the enormous increase in entropy 
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Hmm, barium nitrate isn't too soluble (I don't remember how soluble the hydroxide is). And ammonia would be dissolved in the water, for the most
part. Certainly that's why it makes a paste. I guess most of the entropy comes from freeing the water of hydration and solvating the ammonia.
I've seen that reaction in person, it's quite frosty. 
Tim
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Seems like the CaCl2 method could be a simple replacement for acetone/dry ice for condensing ammonia, methylamine or chlorine.
I wonder how practical this would be in the lab.
Perhaps the barium hydroxide and ammonium nitrate method would get colder if cooled before hand?
[Edited on 5-2-2008 by MagicJigPipe]
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
My CaCl2 solution is in the freezer at -14*C which is about where I want it for the use I have in mind. I used really cheap dessicant from an RV
supplier.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by S.C. Wack
In my crude and small scale experiments using thermocouples, styrofoam cups, CaCl2 hexahydrate from big bag of Prestone ice melt and boiling water,
and snow, the lowest temp that I got was -38C IIRC. This was after cooling the snow and salt to -10C before mixing. Less cooling of the components
produced a corresponding result, it seems that there is just a certain number of degrees of cooling and that's that. So it all depends at what
temperature that you start out at. I tested all of the different ratios stated in the various literature and there was no difference in my hands.
|
In this website they could also get only -37.7 deg. with CaCl2.6H2O (no ratios mentioned), they mention that the literature says -55 deg. is obtainable,
but that even by several attempts they could not get it this cold.
| Quote: | Originally posted by ShadowWarrior4444
What is the mechanism behind the cooling of water in this method? (What does the thermal energy go toward.) |
The difference between salts and the hydrates, is that there is little to no heat from reaction with H2O. An explanation I've seen for the cooling is
that the crystalline structure still requires energy to break down and that warmth from the surroundings is absorbed and so the temperature sinks.
MgSO4.7H2O could also get pretty cold.
Note: the salts "melt" ice, not so much from heat, but because it depresses the feezing point, in other words, it allows the water to remain liquid at
sometimes much colder temperatures.
| Quote: | | Originally posted by 12AX7 I'm amazed KOH is on the list; NaOH becomes very hot when it dissolves! I can't imagine any big difference
between the two salts that would make such a diametrically opposed result. |
I've tried the KOH and ice ratio and it gets pretty cold, colder than ice judging by touch of the beaker.
I tried condensing NH3 gas with it, but it might need some pressure in order for the gas to not escape out of the test tube it was going into and so
that the gas can be brought into contact with the cold much better.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
They didn't say anything about precooling. The lowest temperatures at least with CaCl2 require it. I have no doubt that the lower temperatures can be
attained, but this will require precooling to -20-30C.
I doubt their claim that the cooling with ether and dry ice is ratio-dependent as ether is clearly just a medium for forming a slush bath.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by S.C. Wack
They didn't say anything about precooling. The lowest temperatures at least with CaCl2 require it. I have no doubt that the lower temperatures can be
attained, but this will require precooling to -20-30C. |
No they didn't. Good to know.
| Quote: | | I doubt their claim that the cooling with ether and dry ice is ratio-dependent as ether is clearly just a medium for forming a slush bath.
|
They were just speculating there.
|
|
|
jimmyboy
Hazard to Others
  
Posts: 235
Registered: 1-3-2004
Location: Texas
Member Is Offline
Mood: No Mood
|
|
The list you have doesn't seem like endotherms at all?? - just a list of ionic salt solutions and their freezing points..
maybe I am misreading your question
There is a big difference between solutions with a low freezing point and an endothermic reaction
[Edited on 6-5-2008 by jimmyboy]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
This thread is about endothermic mixtures (achieving lower temperatures), freezing point depression was a secondary but still somewhat related item of
mention.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I've finally found an old reference that mentions the hexahydrate (Römpp's Chemie Lexikon, 7th ed., p. 1677) for -55 deg. CaCl2.6 H2O the ratio given
is 59 g of it to 100 g ice. A few others mentioned here:
BaCl2............22 g/100g ice..........-7.8
NaCl..............26 g/100 g water.....-10
CaCl2.6H2O...71 g /100 g water...-12
NH4SCN........57 g/100g water...-16
(NH4)2SO4.....38g/100g ice......-19
NaBr..............39 g/100 g ice.... -28
MgCl2............22 g/100 g ice....-33
H2SO4 (66.1%)...48g/100g snow...-37
And the CO2: solid CO2 with: alcohol: -72; chloroform: -77; acetone: -86; ether: -100.
They also mention using a Dewar flask (thermos can should work) in order to prepare such mixtures to protect against external warmth influences. Like
one of the webpages emphasizes, there is some variation in the reports of these mixtures. Ether and dry ice got -82.5 by the experimentors in the
previous website. Though they used a basic shallow glass container for cooling, maybe with a Dewar flask it could have gotten even colder.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I've tried the 91 g 66% H2SO4 and 100 g ice ratio. Before adding the acid to the ice, the acid was cooled to -3° in the freezer overnight and the
bottom half of the glass container wrapped in aluminium. After adding the acid to the ice chunks, it got cold within moments and reached the maximum
low temperature of the thermometer (-20°C). I wasn't able to liquify propane with it. After a while the temperature rose to -16°C.
I also added 40 g MgSO4.7H2O to 100 g ice chunks, then stirring to see how cold it would get and the lowest was -8°C. Another 40 g and stirring
brought it down to -9°C. Finally, 70 g more added and it went back up to -8°C. Nothing special that a simple NaCl/ice bath can not reach.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
One of the important bits of information about an ice bath is that the ice should be crushed rather finely and then mixed with the correct amount of
salt. Adding the salt to chucks of ice will work, but is nowhere near as effective.
[Edited on 7-5-2008 by DJF90]
|
|
|
StevenRS
Hazard to Self
 
Posts: 72
Registered: 31-12-2007
Member Is Offline
Mood: No Mood
|
|
I tried the H2SO4/ice mix, and used shaved ice (very fine). It got very, very cold, much colder than my thermometer would go. It condensed SO2 no
problem, and it barely froze isopropanol alcohol. Thats around -90C, I think. Also, the sulfuric and ice were around -13 to start with.
I wonder if you could use different solvents, and different salts to condense liquid air? Use water/salt to freeze a gas like H2S, then use the frozen
H2S (or something else) to freeze another gas, mix it with a salt, and so forth to very, very low temps?
I know the number of steps involved would be large, and some device to hold all the mixtures, each with reducing volume, would be hard (if not
impossible to build) but imagine the rewards!
Liquid O2 made with reusable solvents, salts, and a freezer!
Imagine the fun you could have with that...
Solid ozone... even better....
Any thoughts?
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Here's a result of the ethanol: 105 g EtOH (200 proof, denatured with isoPrOH) mixed with 100 g pounded ice. This mixture went below the maximum
readable low of the thermometer (-20°C), stayed there for a while and later rose. 100 g acetone with 100 g pounded ice also went beyond -20°C, but
it did not stay there as long as the EtOH. Both compounds were precooled in the freezer prior to mixing. Methanol, isopropanol, acetonitrile, or
potentially better even more volatile substances such as tetrahydrofuran, diethylamine, or ether probably yield similar results.
I've also found an old reference called Elements of Chemistry (1842) that mentions precooling also, here they talk about cooling with other freezing mixtures as a form of precooling to
attain the lowest temperatures of the calcium chloride hexahydrate. They also recommend snow or pounded ice.
Another chiller is conc. HNO3 and ice: 1 part HNO3 (60%) with 1 part snow can get as low as -56ºC (source: Photographische Chemie
und Chemikalienkunde (1896), p. 26-27).
From the same source: 1 part of conc. HCl (d= 1.18) and 1 part snow: -37ºC. And also the sulfuric acid: 1 part H2SO4 (diluted by some water), 3 parts
snow: -50ºC. 5 parts crystallized CaCl2 and 4 parts snow: -50ºC.
Moderatley diluted H2SO4 when poured onto snow can reach a low of -40 to -50ºC (Grundriss der Experimentalphysik und Elemente der Chemie (1896), p.
253).
From another source (Lehrbuch der klinischen Osmologie als funktionelle Pathologie und Therapie (1902), p. 301): 1 part by mass water and 1 and ½
parts by mass powdered KSCN reaches -34.5ºC. For comparison: 1 part snow and 1 part NaCl (by mass): -21.3ºC. 1 part snow and 3 parts crystallized
CaCl2 (by mass): -33 º. 1 part by mass of snow and 1 part by mass of diluted H2SO4: up to -50º.
And lastly, from another reference (Lehrbuch der chemischen Technologie der Energien (1906), p. 227-8): 2 pbw (parts by weight) dilute H2SO4 with 3
pbw. of snow or ice: -30 deg.; 5 pbw. NaCl and 5 pbw. NH4NO3 with 12 pbw ice or snow: -32ºC; 10 parts H2SO4 (65.3% conc. at 0 deg.) with 11 parts ice
or snow: -37.0 deg.C.; 1 part HCl acid (spec. gravity: 1.18 at 0 deg.C.) with 1 part snow or ice: -37.5 deg.C.; 4 parts potash with 3 parts ice or
snow: -48.5ºC; 1 part HNO3 (60% N2O5 at 0 deg.) with 2 parts ice or snow: -56.0 deg.
There were also some others, like 6 pbw. Na2SO4 with 4 pbw NH4Cl and 2 pbw. KNO3 solubilized in 4 pbw of dilute HNO3 goes from +10 to -23ºC or 6
parts by weight (pbw) Na2SO4 and 5 pbw NH4NO3 solubilized in 4 pbw dilute HNO3 goes from +10 deg. to -40 deg.C., etc.
More specs on the hexahydrate:
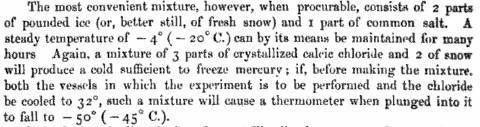
From: Elements of Chemistry (1877), p. 368.
This following is from an older CRC handbook about the proportions of the hexahydrate or acid:
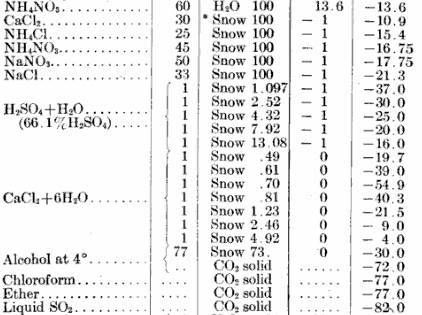
Gathering from that, it seems like that what should be used for the hexahydrate at least is snow instead of ice (* or finely pulverized ice); ratio
for the -55º: 1 to 0.70, thus 143 g added to 100g snow. So far everyone’s suggestions have been excellent (e.g. precooling, pulverized ice, SO2
testing).
| Quote: | Originally posted by StevenRS
I tried the H2SO4/ice mix, and used shaved ice (very fine). It got very, very cold, much colder than my thermometer would go. It condensed SO2 no
problem, and it barely froze isopropanol alcohol. Thats around -90C, I think. Also, the sulfuric and ice were around -13 to start with.
|
Did it liquify or solidify? If it only liquified then it reached below the B.p. of -10°C, however if it solidified that would mean it went below
-72.4°C (M.p. of SO2). SO2 would probably be the best way to test for lower temperatures because it easily liquefies, and then if it gets colder will
solidify.
| Quote: | I wonder if you could use different solvents, and different salts to condense liquid air? Use water/salt to freeze a gas like H2S, then use the frozen
H2S (or something else) to freeze another gas, mix it with a salt, and so forth to very, very low temps?
I know the number of steps involved would be large, and some device to hold all the mixtures, each with reducing volume, would be hard (if not
impossible to build) but imagine the rewards!
Liquid O2 made with reusable solvents, salts, and a freezer! |
I think it's in the realm of possiblity to find a mixture at the extreme end of endotherms as there are in exotherms - and that's what this thread is
all about, but these seem to me to have been less studied. Thermochemistry seems more interested in the hotter spectrum.
| Quote: | Imagine the fun you could have with that...
Solid ozone... even better....
Any thoughts? |
Imagine being able to make your own dry ice. 
[Edited on 8-5-2008 by Schockwave]
|
|
|
StevenRS
Hazard to Self
 
Posts: 72
Registered: 31-12-2007
Member Is Offline
Mood: No Mood
|
|
Neither became truly solid, the SO2 was not cooled as well at all as the Isopropanol, which started to become very viscous, and started to freeze.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I've done a couple more. First, 20 g of finely powdered NaOH was added to 90 g of finely crushed ice in a beaker wrapped in Al foil, then quickly
stirred. This mixture would go about to -10ºC, but not below. Next, the same was done except 20 g powdered KOH was added to 90 g of finely crushed
ice; this went as low as -16ºC. Neither hydroxides were precooled.
Not last but not least, 100g of around 54% HNO3 (precooled in the freezer) was added in the same manner to 100 g of finely crushed ice. By adding the
acid, the temperature went immediatley below the maximum readable of about -20ºC. Later it rose to about -20ºC, and more.
And lastly, an addendum to the ethanol: adding 100 g pounded ice to 105 g ethanol (denatured) which was not precooled in a beaker got no lower than
about -18ºC.
[Edited on 8-5-2008 by Schockwave]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Condensing could take a while so it might not be very accurate for determining how cold a mixture gets beyond a thermometer. Even about 3.5 mL 99%
isopropanol in a glass tube put into around 200 mL liquid N2 (-196 deg.) took around over 1 minute to become solid. Right after melting (m.p.
-89.5°C) the alcohol was a thick viscous liquid, similar to glycerin in consistency.
Liquid N2 freezing mixtures (and the source):
hexane / liquid nitrogen: -94 deg.
methanol / liquid nitrogen: -98 deg.
pentane / liquid nitrogen: -131 deg.
I've tried the methanol/N2 one as it's used to chill chemical reactions. When the liquid N2 is in excess the methanol is left behind as a frozen solid
after the nitrogen has boiled off. When methanol is in excess then the N2 boils off and dances on it, similar to water. Methanol vapors also get
pushed out of the container in the process.
|
|
|
nodrog19
Harmless

Posts: 38
Registered: 29-5-2008
Member Is Offline
Mood: No Mood
|
|
i found this table in Organic Laboratory Techniques, 3rd Edition
ice- water -0C
100g ice 33g NaCl -21C
70g ice 100g CaCl2*6H2O -54C
dry ice- ethanol -72C
dry ice- acetone -77C
|
|
|
| Pages:
1
2 |