| Pages:
1
2 |
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
There's been some more talk on freezing mixtures over at the German forum, so I've decided to add some more information to this thread. Disregard the
improbable values for MgCl2 and H2SO4.
More clarity on the acid mixtures comes from Gmelin: 3 parts snow and 1 part sulfuric acid containing 1/5 its mass of water reaches -32.5°. If the
materials are precooled to -7°, then the temperature sinks to -51°. Since the 18th century similar mixtures of snow and dilute H2SO4 have been used
to freeze mercury. Nitric acid mixes have also been used to freeze mercury. HNO3 with 1.5 H2O when mixed with two times its mass of snow, starting at
0 deg. causes a temperature depression of -56°.
A corroboration reference for mixing of snow with absolute alcohol causing a depression of 0° to -30° is Handbuch der biochemischen Arbeitsmethoden
by E. Abderhalden. If precooling in a freezer, I feel the need to reiterate never to leave a flammable unstoppered, so no explosion can result.
Concerning the mixture of ether and dry ice. After looking through old literature, this one was a favorite freezing mixture. Being able to reach
temperatures occasionally colder than the dry ice itself, and a drop or two of the mixture is said to be able to produce blister burns on the skin.
But not normally reach near -100°.
In Gmelin it's stated that Faraday with solid CO2 and ether got -77°, for some strange reason B. Schwalbe got a wide range, from -77 to -97°.
Anyways, I think the value of -100° might actually come from Faraday achieving -103° using an airpump for the same mixture, and Schwalbe reaching
-110° also using an air pump. E. Abderhalden states solid CO2 and alcohol in a vacuum causes the temperature to sink below -100°. The increased
evaporation being responsible for the heightened cooling.
I oversaw the reference earlier in Elements of Chemistry, stating some guy named Walker did experiments using freezing mixtures against other freezing
mixtures to cool even further. With 8 parts snow and 10 parts dilute H2SO4 (I'd like to know exactly how dilute) when frozen (using CaCl2-mixes) goes
from -54.4° to -68.3°. A sulfuric acid-water freezing point curve is below.
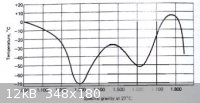
[Edited on 9-11-2009 by Formatik]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
The ratio
A system of chemistry, Vol.1 by J. Murray, who seems to have seen the original reference of Walker, states the ratio is 10 parts sulphuric
acid diluted with half its weight of water, with 8 parts snow. In total, this is about 28% conc (d= 1.20). What this mixture has reached, by the old
literature, it is said to have been the coldest temperature measured (well, without dry ice, liquid gases, compression devices).
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
A type K thermocouple (the most common type AFAIK) is good down to liquid air temperatures, and the chromium-nickel alloys are poor heat conductors.
Many inexpensive (less than $US 50) multimeters come with a bead thermocouple with teflon insulated wire. Such a system is very useful to measure
cooling mixtures. A sleeve made of thin teflon tubing closed at one end could protect the thermocouple from salts & acids. To make such a sleeve,
heat some teflon tubing until it softens and starts to become transparent or translucent. Remove it from the heat and squeeze it gently but firmly
closed with pliers, a clamp, or a vise. Don't squeeze it to a thickness below about 1 1/2 wall thicknesses. The tubing will fuse together giving you a
condom for your thermocouple.
When snow falls where I live I'll experiment more with freezing mixtures... the Canadian members should have access to some good OTC chemicals for
this purpose 
I have some teflon tubing with ID about 1/8" (3 mm or so) which I would be willing to cut to 10-30 cm lengths and mail to people for postage + a
trivial amount.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Formatik, using snow which I feel is essential due to the orderness of bonding being broken all at once, never once reached temperatures no matter
what concentration I tryed. HCl and snow would out do H2SO4 everytime reaching -40s IIRC(I have a thread here or at science forums somewhere). I tryed
shaving ice but got nothing. I attempted to condense ammonia with the HCl/snow mix but got limited results. I was hoping someone was able to have
success with CaCl2 hydrates as this looked very nice indeed.
Might I suggest if you get snow before me to attempt a concentrated solution of CaCl2 on snow instead of trying to get the two powders to mix quick
enough. I have had a strong suspicion all year that this may achieve the results documented.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by densest  | | A type K thermocouple (the most common type AFAIK) is good down to liquid air temperatures, and the chromium-nickel alloys are poor heat conductors.
Many inexpensive (less than $US 50) multimeters come with a bead thermocouple with teflon insulated wire. |
Unfortunately, I found out about thermocouples and multimeters too late. 
| Quote: | | Such a system is very useful to measure cooling mixtures. A sleeve made of thin teflon tubing closed at one end could protect the thermocouple from
salts & acids. To make such a sleeve, heat some teflon tubing until it softens and starts to become transparent or translucent. Remove it from the
heat and squeeze it gently but firmly closed with pliers, a clamp, or a vise. Don't squeeze it to a thickness below about 1 1/2 wall thicknesses. The
tubing will fuse together giving you a condom for your thermocouple. |
Be careful heating teflon, since very toxic gases especially perfluoroisobutene are released if it's overheated/decomposed.
| Quote: | When snow falls where I live I'll experiment more with freezing mixtures... the Canadian members should have access to some good OTC chemicals for
this purpose  |
If no snow is available, one could also try with pounded, pulverized ice that isn't wet.
Quote: Originally posted by Sedit  | | I attempted to condense ammonia with the HCl/snow mix but got limited results. I was hoping someone was able to have success with CaCl2 hydrates as
this looked very nice indeed. |
For something like liquefying ammonia, the literature says to use large amounts of cooling mixture. For saftey reasons, the CaCl2 hydrate might be the
better idea.
| Quote: | | Might I suggest if you get snow before me to attempt a concentrated solution of CaCl2 on snow instead of trying to get the two powders to mix quick
enough. I have had a strong suspicion all year that this may achieve the results documented. |
There's an interesting reference I've found on this: Über die Kältemischung aus Chlorcalcium und Schnee (= concerning the freezing mixture
of calcium chloride and snow) by Dr. Hermann Hammerl. (from: Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Vol. 78, Pt. 2. 1879. Pgs.
59 to 79). This is a somewhat lengthy paper on just the cooling mixture of calcium chloride and snow alone. I have seen quite some variation when it
comes to the ratio of the mixture, but this paper is well detailed. So I trust the ratio given there which is: 1 gram CaCl2.6H2O with 0.70 gram of
snow, for lowest cooling value and temp. of -54.9°C. On pg. 74, and of this temperature he says: "in order to always reach this temperature minimum
from mixing snow and calcium chloride, most importantly the crystallized calcium chloride is in a finely powdered form, and if possible be cooled
below 0°C. The snow should not be wet, but crumbly, and also cooled below 0°C." He said that he and his colleges - through experimentation - always
got below -50°C when mixing in the proper proportions.
|
|
|
nickd
Harmless

Posts: 1
Registered: 4-1-2007
Location: Sussex Uk
Member Is Offline
Mood: No Mood
|
|
Freezing point depression
Whilst there is some some variation in freezing point depression with different substances, you may remember that freezing point depression is a
property that dependes on the RATIO of the solute to the solvent particles rather than the nature of the actual substance itself
.The depression effect of freezing point changes are related to the number of particles and does'nt involve solute /solvent interaction. (It is
related to the molality of the solutions as represented by Raoult's law)
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
These mixtures involve several phenomena. For one of the reasons why the temperature drop occurs in the chloride mixture- the enthalpy of solution -
solvent-solute interactions do have an influence.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Nickd: You are *partially right*, in that the depression effect of freezing point (and also temperature dependance of solubility) are related the the
ratio of the solute/solvent (i.e. the mole fractions). However, this is only true when you consider the system as an *ideal* solution; one where the
enthalpy of mixing is zero, and the gibbs free energy relies only on the entropic term.
However, this is only a model. A step up would be to treat it as a "regular" solution, which has an "ideal" entropy of mixing and a non-zero entalpy
of mixing.
Even better would be to use the "electrolyte" solution model, which is based on Debeye-Huckel theory.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
just a stupid thought i always had regarding ice salt baths, what would happen if the ice and salt you were combining were already at a temperature
below -31. Would the system warm to -31, perhaps this is where an intelligent design advocate needs to chime in and let us know.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I tryed adding finely crushed CaCl2 from damprid that had been moistened and sat out for a while till it reclumped where it was grounded down again
till it reclumped.
I ground it once more and added it to a bowl full of fine ice. It use to be snow but its been sitting in the cooler for a week for I guess its best to
just say fine ice at this point because thats how it would behave where as snow would get alot lower I believe.
Anyway one with the shitty data. I did not keep all numbers since im just playing but after the addition the lowest temperature I was able to achieve
was -31C. I now see that judging from the chart up there that importance over the amount of CaCl is a must so I guess I will take better measurements
next time. My main goal was to test the temperature since I want to rerun the Li[NH3]4 experiments and thats seems cold enough to be effective. I just
didn't want to mess around with another HCl/Ice bath for obvious reasons. It was good that at one point there was small leak in the NH3 generator and
the small amounts of HCl around quickly showed it but I still want nothing to do with a bowl of HCl even if its been diluted.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
An Unusually Philosophical Post
By Mr. Richard Walker:
Observations on the best Methods of producing artificial Cold.
Phil. Trans., 270 (1795)
On the Production of artificial Cold by Means of Muriate of Lime.
Phil. Trans., 120 (1801)
Attachment: phil_trans_270_1795.pdf (1.8MB)
This file has been downloaded 903 times
Attachment: phil_trans_120_1801.pdf (757kB)
This file has been downloaded 834 times
|
|
|
Random
International Hazard
    
Posts: 1018
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
How low the temperatures could go if I would put NaCl in freezer at -20 and take it out after 2 days and then mix it with -10 ice. Could it go below
-20 then?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Freezing mixtures generally do not go colder than the eutectic, and the NaCl-H2O eutectic point is −21 C.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by S.C. Wack  | By Mr. Richard Walker:
Observations on the best Methods of producing artificial Cold.
Phil. Trans., 270 (1795)
On the Production of artificial Cold by Means of Muriate of Lime.
Phil. Trans., 120 (1801) |
Wow, that's pretty interesting. What Walker is saying in the second document is that below -91 has been reached, being held there for 20 seconds. The
-68 mixture was also actually 8 parts conc. H2SO4, 4 parts water, 1 part grain ethanol cooled to air temperature. Then he took 10 parts of this mixed
acid, mixed it with 8 parts snow and chilled it with the CaCl2 mixture. Walker is probably some kind of original source for the idea H2SO4 and ice
gets -90 C, mentioned on the first post in this thread.
Also I think interesting, the effect of precooling on calcium chloride mixtures, described on p. 133. It doesn't matter if snow or ice powder are used
either, they reach the same temperatures in the mixtures.
I haven't had much time for experimentation, but one thing I did do a while back was to condense propane with dry ice. Then adding dry ice to the
propane, and attempted to freeze 91% isopropanol, but it only got viscous after a couple minutes. The liquid propane was dangerous to handle not only
because of flammability and stench, but because it causes severe burns. A drop or so of the liquid propane on my hand caused a severe frostbite burn
that took months to heal. I thought since it was claimed SO2 and dry ice get below dry ice itself, if I were to take something more volatile it could
yield similar or even better results.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I found that mixing dry ice with iPrOH, acetone, pentane and diethylether all stops at -78C. There seems to be no difference whatsoever.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | One of the important bits of information about an ice bath is that the ice should be crushed rather finely and then mixed with the correct amount of
salt. Adding the salt to chucks of ice will work, but is nowhere near as effective.
[Edited on 7-5-2008 by DJF90] |
Yes, absolutely: fineness of the mixtures is everything.
Another very common exothermic reaction: neutralisation of NaHCO3 with any strong acid. An entropic effect apparently due to the escaping CO2...
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Some more action from me. I've quantified it well compared to last attempts from some time ago. I repeated the mixture of dry ice and liquid propane
mixed in a plastic thermos can, and after mixing them I got a reading of -65 C (Note: I condensed the propane with some dry ice, and then added some
more dry ice). It didn't get much colder than that for some time, so I used an aquarium air pump set on high and used a tube to bubble air through it,
and this caused the temperature to drop rapidly. After maybe something like 30 minutes of bubbling, I was able to eventually get -80 C. But, the same
can be done with acetone.
I did the same thing using acetone. 158g solid CO2 pieces and 102g acetone when mixed in a plastic thermos can started out at -64 C, and it only
decreased slowly after one minute to -65 C, then another minute to -66 C. So, then I bubbled in air with the same pump and this promoted temperature
drops. The decrease was by 3 C within about a minute, then by 1 C per minute for 6 minutes to get to -75 C. Then after bubbling in air an additional
17 minutes, the temperature eventually dropped to -80 C. Then the reading was fluctuating slightly between -81 and -80 C, and it was like this up to
18 more minutes, after which I added 10g X 2 dry ice pieces as I kept bubbling an additional 13 minutes after dry ice addition, after which time it
then reached -83 C. After 5 minutes, I added 28g solid CO2 during the bubbling. And then temperature readings started fluctuating and increasing. But
I then removed the air pump. And took the multimeter (it was on the cold concrete ground of the side garage). As I moved it to a warmer area in the
house, I noticed colder readings that went below -83 C, I later took the air pump to this mixture and just let air hit the surface/go over the liquid
instead of bubbling in, and I was able to get steady readings of maximally -88 C, some fluctuations went below but these were due to moving the
multimeter during measurement.
I used a miserable air pump source. I think a better pump or even a vacuum, ought to produce promised literature values.
I wasn't sure if I were to be able to get colder than dry ice (-78 C) alone at first, but the trick to cool down dry ice freezing mixtures faster by
blowing air through it seems to work pretty well, this could be useful to cool those kind of mixtures much more rapidly.
Liquid nitrogen can possibly be made colder on similar principle by blowing anhydrous helium or hydrogen through or over it.
Some pictures are below.

Liquid propane mixed with dry ice and air bubbling in.

Propane and dry ice multimeter reading.

The set-up of acetone and dry ice.

Acetone and dry ice after bubbling air through it for under 30 minutes.

Steady cold readings.
[Edited on 18-12-2010 by Formatik]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I'm not sure if it has been pointed out in the thread thus far, but to achieve these really low temperatures using frigorific mixtures, it is
important that they are being cooled by a supplementary source.
| Quote: | | These artificial processes for generating cold are much more effectual when the materials are previously cooled by immersion in other
frigorific mixtures. |
- Elements of Chemistry
This means, for instance, that the sulphuric and ice needs to be sat in a second container of say, KOH and ice. I have tried the sulphuric method
three or so times before, with it sat in a dewar, and never been able to get the temperature below that of the ice when it came out of the freezer.
I tried again today with KOH and ice, as follows:
A Thermos, it's inner and outer cap were placed in the freezer, along with 100g of crushed ice and 31g of KOH (all kept as separate components). They
were removed hours later, when the inside of the flask was reading -20C. The thermometer was poked to the base of the Thermos, the cold, measured 31g
of KOH dumped in, then the 100g of crushed ice, fresh out of the freezer. I put the cap back on and gave it a good shake, checked the thermometer was
at the base and replaced both the inner and outer caps.****
I then sat and wrote down the temperature on a minute by minute basis for half an hour.%%%
These are the values I obtained (in Celsius), per minute:
-20 (0 minutes, freezer temperature)
-14
-3
4
7
2.8
-0.6
-3.2
-5.4
-6.8
-8.6
-9.8
-10.8
-11.9
-12.6
-13.5
-14
-14.5
-14.9
-15.5
-15.8
-16.1
-16.4
-16.6
-16.8
-17.2
-17.3
-17.5
-17.6
-17.6
-17.6
As the trend was now stabilising, I left it for much longer periods of time. Here are the results that occurred:
45 minutes
-18.7
66 minutes
-17.7
75 minutes
-17.4
158 minutes
-16.9
207 minutes
-15.5
226 minutes
-15
321 minutes
-12.7
423 minutes (some 7 hours after combining the two)
-10.2
The mixture rapidly rose in temperature when shaken together, then (soon after) fell in a de-accelerating curve for half an hour. From there, it has
sat in a very gradual linear rise back to room temperature. I've included the numbers such that you can plot them yourself in Libre / Open / Microsoft
Office if you so wish.
Enthalpy of fusion (water): 6.01 kJ/mol
Enthalpy of solution (KOH): -57.61 kJ/mol
This is a 31g / 100g KOH to ice mix, as specified in the table, and measured on a digital kitchen scale (reasonably accurate for this task).
100g of H2O: 5.6 molies
31g of KOH: 0.55 moley moles
Enthalpy of fusion for 100g H2O: 33.6 kJ
Enthalpy of solution for 31g KOH: -31.6 kJ
Those numbers are close, the KOH is giving out almost as much heat as the ice absorbs in melting (based on their normal melting and dissolving figures
as separate entities). The mixture as a whole, based on those values, gives a total heat absorbing capacity of 2kJ, versus the 5.6 kJ the ice alone
would have had at 0C, melting on it's own; 2 to 3 times less.
From the graph, it is clearly evident that the test is not wanting of patience, as the linear rise means the process is now complete - there will be
no more cooling. I wanted to point this out as, when I first saw the numbers in the table, I was optimistic I could have some fun with those, in a
practical sense. However, the process is not particularly applicable to practical work (e.g. running condensers or baths), because those baths do need
stacking. With the mixtures I've tested not even being able to get below -20C, they are actually performing worse than regular table salt / ice baths,
and using more expensive and corrosive depressants. On top of that, my freezer can manage -30C. And, on top of that, the baths (unlike dry ice and
LN2) do not just need topping up, they need emptying and refilling (not something you'd want to be doing if cooling something that is going to go
bananas when the bath / condenser is restacked).
Following the logic of bath stacking, with the freezer at -30C and the baths sat in there, one could start with the two CaCl2 baths and then stack
into these the KOH bath. I suspect that would reach (or at least get closer to) the specified -63C.
I believe these mixtures were originally developed more as temperature reference standards, perhaps for applications such as calibrating thermometers.
I commend the original researcher, but they not a substitute for dry ice where heat loads must be absorbed.
I also believe that a much more profitable expenditure of time would be to simply freeze an ethanol mixture in the freezer, to gain a -30C slush.
There are some long chain alcohols, I can't remember which, that solidify as native species in this temperature region, and absorb a fair amount of
heat in their state change (I think I found these on matweb or in the Rubber Bible, the CRC book). There are also metal alloys that may be of some
amusement.
***Photos
%%%Graphs. Image shack just imposed a 500 photo limit on their free accounts. As mine had over a thousand in it, quite a few of my photos have been
replaced with 'violated rule' tags. I've uploaded these as rough pictures, I don't know how long those will stay on there.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by peach  | | I'm not sure if it has been pointed out in the thread thus far, but to achieve these really low temperatures using frigorific mixtures, it is
important that they are being cooled by a supplementary source. |
It was mentioned in several places, but yes, precooling is important and crucial to obtaining literature lows with frigorific (endothermic) mixtures.
I've also found this to be the case.
It was also mentioned the effect of increased evaporation being able to cool the mixture even more by blowing air through it or using a vacuum. Though
hasn't been discussed with relevance to endothermic mixtures (just freezing mixtures involving dry ice). But I have been able to cool a regular
hydrated MgCl2-ice mixture a few degrees more just by blowing air through it.
| Quote: | I tried again today with KOH and ice, as follows:
A Thermos, it's inner and outer cap were placed in the freezer, along with 100g of crushed ice and 31g of KOH (all kept as separate components). They
were removed hours later, when the inside of the flask was reading -20C. The thermometer was poked to the base of the Thermos, the cold, measured 31g
of KOH dumped in, then the 100g of crushed ice, fresh out of the freezer.
....
45 minutes
-18.7 |
I got something similar, but slightly warmer using a mixture of: 20g fine KOH and 90g finely crushed ice reaching -16 C, when neither were precooled.
| Quote: | | Following the logic of bath stacking, with the freezer at -30C and the baths sat in there, one could start with the two CaCl2 baths and then stack
into these the KOH bath. I suspect that would reach (or at least get closer to) the specified -63C. |
Right, the data is not so straight-forward as it as been put on some tables. I also think the KOH mixture could reach that low by cooling it with
other freezing mixtures. Though I have pulverized KOH in one of my cooling experiments and that was no fun. Extremely irritating in dust form.
I suspect if there's any truth to it (the only reference I think I've seen for it is the webpage first mentioned in this thread), that this is the
case for MgCl2.6H2O-ice mixtures being able to reach the purported lows. In which case it would have to be cooled with other cooling mixtures, or
basically just dry ice to potentially reach temperatures below that of dry ice.
| Quote: | | I believe these mixtures were originally developed more as temperature reference standards, perhaps for applications such as calibrating thermometers.
I commend the original researcher, but they not a substitute for dry ice where heat loads must be absorbed. |
This has been more of an exploratory thread for the sheer experimentation and knowledge. But if I was to use a cooling mixture my first choice would
be a cheap salt and ice (or snow), dry ice with or without solvent (then air blown through it to cool it down faster and better), or liquid nitrogen.
|
|
|
| Pages:
1
2 |
|