amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
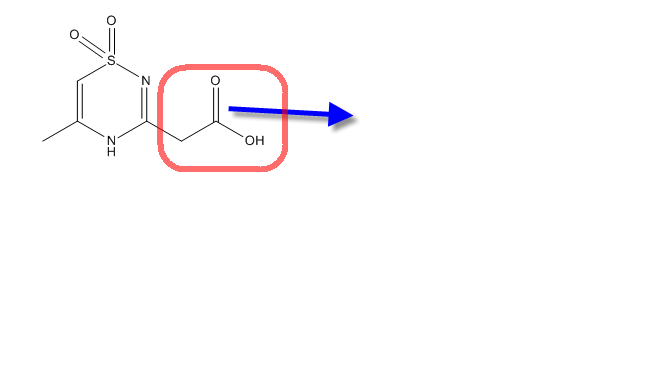
thiadiazine dioxides
I am looking for ideas for the synthesis of this heterocycle
Literature search in post number 3
[Edited on 11-5-2008 by amrhamed2]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Did you check the literature? Done some research first? Also what is the red box supposed to indicate?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
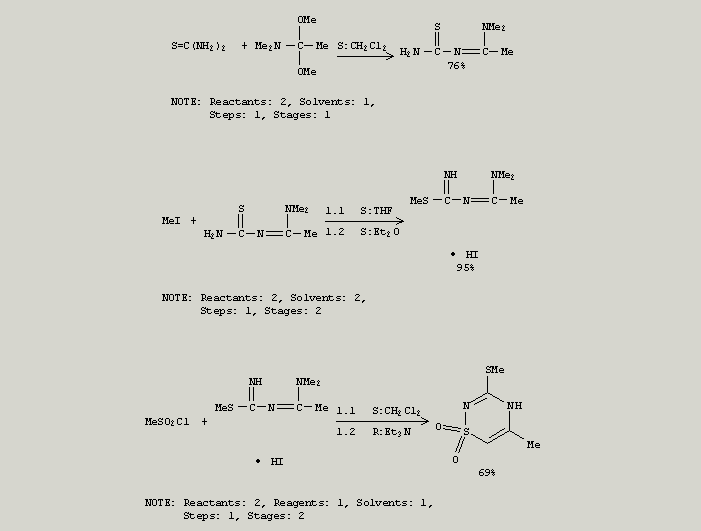
I have searched for a method and here is an idea which i wanted to discuss with u
The idea is dependent on N,N-Dimethylacetamide dimethyl acetal
I have abstract for this paper (if somone have access to the paper ,plz upload it)
Cationic 1,3-diazadienes in annulation reactions. Synthesis of pyrimidine, thiadiazine dioxide and triazine derivatives.
Landreau, Cyrille; Deniaud, David; Reliquet, Alain; Reliquet, Francoise; Meslin, Jean Claude. Laboratoire de Synthese
Organique, UMR CNRS 6513, Faculte des Sciences et des Techniques, Nantes, Fr. Journal of Heterocyclic Chemistry (2001),
38(1), 93-98. Publisher: HeteroCorporation, CODEN: JHTCAD ISSN: 0022-152X. Journal written in English. CAN 135:33464
AN 2001:223206
Triazapentadienium iodides Me2NCR1:NC(SMe):NH.HI [R1 = H, Me], prepd. from Me2NCR1:NCSNH2, are efficient intermediates
in heterocyclic synthesis. They react with ketenes, sulfenes, Ph isocyanate or isothiocyanate and di-Me acetylenedicarboxylate
affording dihydropyrimidinones, thiadiazine dioxides, triazinones, triazinethiones, and pyrimidines.
It is easy to substitute the SMe in the final compound .....??Isn't it????
[Edited on 11-5-2008 by amrhamed2]
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
weird enough you have access to scifinder but not to the paper...
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
weird enough you have access to scifinder but not to the paper... |
That is very possible. Scifinder is only a reference database, you don't get any journal subscriptions with it. Let's get back on topic now.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
nobody answered me .....
Can I easily convert SMe into CH2COOH ??? (I think it can be easily done via dimethylmalonate and sodium ethoxide followed by decarboxylation)
[Edited on 14-5-2008 by amrhamed2]
[Edited on 15-5-2008 by amrhamed2]
amr h mahmoud
|
|
|