| Pages:
1
2 |
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
My experience with phenol was with it's magnesium salt, during the formylation with Mg(OMe)2/(H2CO)n. No dissolved oxygen as the magnesium methoxide
was generated with magnesium metal, reducing any dissolved oxygen and saturating the suspesion with H2 I suppose, and all the added solvents were
degassed under vacuum/argon cycles.
Of course, the conditions were surely a little rougher than RT in water , as it involved distn and several hours reflux in toluene, when the
recation was done under atmospheric conditions, the phenolate was definatively darker/browner than when argon was used. The magnesium counter-ion
could be responsible in part for easier degradation, i suppose.
For the alkylations, I've always considered 3x vacuum/argon cycles to degass the solvent pretty effectively, especially with DMF which tolerates a
higher vacuum before ebullition.
But I guess the oxidation occurs to a very small extent, and that it shouldn't be to detrimental to the yield. Just to perfectionist (or nit-picky
 ) I guess ) I guess  It's just that having the vacuum and argon at hand it doesn't cost much to use it, and it's routine at work. I admit it isn't be mandatory here. It's just that having the vacuum and argon at hand it doesn't cost much to use it, and it's routine at work. I admit it isn't be mandatory here.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
I don't think the oxidation by-products are a big deal with HQ anyway, although for more sensitive substrates precautionary measures could be taken.
Venting the solvent/flask with MeBr should work well too (for those that haven't access to Ar).
Using other solvents definitely is a good idea as well - I just used what I had on hand (my isopropanol is 91%, although that should also work fine).
Isopropanol might provide a neat workup though, as one can make the 2-methoxypropane and extract with it (granted a bit of extra MeBr would then need
to be used)! It boils at 32*C, so after diluting the RM and separating the layer, one can just let it sit and evaporate, which after a few minutes
should leave very pure and dry product.
[Edited on 20-5-2008 by PainKilla]
|
|
|
Brainiac
Harmless

Posts: 5
Registered: 7-6-2008
Member Is Offline
Mood: No Mood
|
|
Hi,
Recently I have been trying to form 1,4 Dimethoxybenzene via methylation of Hydroquinone with Methyl Iodide.
0.1M HQ was dissolved in 75ml Acetone. 0.2M NaOH was added followed by the dropwise addition of 0.2M MeI.
The reaction was kept at reflux for 6 hours. During this time, the entire contents of the reaction mixture turned black.
50ml. of the Acetone was then Vacuum distilled off and the residue was added to ice water. This precipitated out black and white crystals. When vacuum
filtered, they took upon an irridescent appearence.
Could anyone suggest what these crystals might be?
I look forward to hearing your views on the subject.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
.....1,4-dimethoxybenzene?
Using MeI, especially with such ane xcess, seems wastefull. I would advise preparaing p-methoxyphenol using MeOH/H2SO4/benzoquinone, then alkylating
that with MeI, using stoechiometric amount of base, the 100% exces NaOH you used surely degraded half your MeI... Thsi takes more works howeevr.
I would use a salt of methylsulfuric acid as reported previously, very cheap satrting material and no toxic and cancerigenic product....
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Brainiac
Harmless

Posts: 5
Registered: 7-6-2008
Member Is Offline
Mood: No Mood
|
|
Surely the 0.2M Methyl Iodide is required to methylate both the 1 and the 4 position in the first place? And then the 0.2M Sodium Hydroxide to react
with the 0.2M Hydrogen Iodide that would be produced?
Or am I just way off with my thought trends here?
Thanks
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Oh! My bad sorry I had the p-methoxyphenol alkylation in head 
You a absolutly right, 2 equivalents are needed, sorry for the confusion 
Why do you doubt the crystasl are your product? try taking a mp after recrysatllization would confirm, but it seems you obtained the wanted product.
What was the yield like?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Brainiac
Harmless

Posts: 5
Registered: 7-6-2008
Member Is Offline
Mood: No Mood
|
|
Well, I have yet to weigh the yeild because I havent had a chance to get back to the lab. However from what I have collected through filtration (the
black pearlescent solid) the crystals seem distinctivly not white (as most MSDS for 1,4 Dimethoxybenzene state).
There is, however a very pleasent smell that I believe could be attributed to the reaction product. It certanly is not similar to Methyl Iodide- or
infact Benzoquinone.
Will take a melting point test and quantify the yeild later today and report back.
Regards,
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
The black colour is du to quinonic impurities trapped in the product, that black colour your reaction medium had when the abse was added. A simple
recrystallization should get rid of it.
Indeed, dimethoxybenzene has a pleasant smell, so I guess that's what you've obtained.
But again I would suggest using somethign else than MeI which is a very noxious compound, and when you say the product doesn't smell like MeI, I hope
you didn't take a sniff from your bottle!
I would go by the methyl sulfuric salt method, even commercial dimethoxybenzne is much cheaper than MeI.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Brainiac
Harmless

Posts: 5
Registered: 7-6-2008
Member Is Offline
Mood: No Mood
|
|
MeI, while normally is expensive, I only pay £5 for 100ml. so cost is not exactly an issue.
Though I do see your point, 1,4 Dimethoxybenzene is rather cheap- though the lab skills have been invaluble.
MeI is not that awfully noxious- I wouldnt reccomend drinking it, but in comparison to some things (cyanide etc) it really isn't that bad.
Thanks. I'll water wash the product and quantify yields.
Regards,
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
I'd work with cyanides any day over working with methyl iodide. It's carcinogenic, toxic - both acute and as a mutagen, and on top all that: volatile!
If I were you, I'd reconsider your position on methyl iodide toxicity, as carcinogens are among the few chemicals that will come back to bite you
after an unknown period of time.
Try dissolving your solid in a bunch of NaOH/methanol, and then dump that mess into 10x the volume of water, the dimethoxybenzene will crystallize
out.
|
|
|
Brainiac
Harmless

Posts: 5
Registered: 7-6-2008
Member Is Offline
Mood: No Mood
|
|
Not to get picky... Methyl Iodide is Cat 3 carc. And just because I said that its relatively not too toxic- doesnt mean to say that I use it
haphazardly!
Fume Cupboard, nitrile gloves, lab coat and face sheild/respirator, after having refridgerated the bottle for several weeks to reduce volitility!
Thanks very much however for the concern and for your advice with regards the NaOH MeOH. Its very much appreciated.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Brainiac
Well, I have yet to weigh the yeild because I havent had a chance to get back to the lab. However from what I have collected through filtration (the
black pearlescent solid) the crystals seem distinctivly not white (as most MSDS for 1,4 Dimethoxybenzene state).
There is, however a very pleasent smell that I believe could be attributed to the reaction product. It certanly is not similar to Methyl Iodide- or
infact Benzoquinone.
Will take a melting point test and quantify the yeild later today and report back.
Regards, |
I own 2 500 gm bottles of HQ dimethyl ether. The product is white & the smell is pleasant. See http://en.wikipedia.org/wiki/1,4-Dimethoxybenzene
[Edited on 6-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
1,4-dimethoxybenzene can be alkylated with tert-butyl alcohol in acetic acid using sulfuric acid as a catalyst. The product forms nice crystals. The
procedure can be found in newer editions of the Williamson textbook in the Friedel-Crafts reaction section. I ran this synthesis in o-chem lab last
year. I am interseted in preparing some 1,4-dimethoxybenzene using the bromomethane method as I have 250g of hydroquinone. I prepared
1,4-diethoxybenzene once using bromoethane, but the product was dark in color and was obtained in low yield.
Amateur NMR spectroscopist
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
I'm currently running a veratraldehyde synthesis using the MeBr method that PainKilla described in one of his posts in this thread. It's on smaller
scale though (70 mmol vanillin) but I'm hopeful that it will be a success. 
I'll post a writeup when I finish the reaction and work it up, it's currently in the MeOH/H2SO4 addition phase.
A picture of the general setup is found in the attachment. Washing bottle was filled with 10% ammonia in water (just to the inlet tube).

A quick note: Be careful on the addition rate of the MeOH/H2SO4 solution (in this and all gas generation reactions ofc.)! I succeeded in testing the
gas tightness of my apparatus by being sloppy on the addition funnel tap and managed to blow open the receiver I'm using as the overpressure "valve".
 No harm done though (using a proper full face gasmask with ABEK filter when
working, no fumehood No harm done though (using a proper full face gasmask with ABEK filter when
working, no fumehood  ) , just scared my self a little. ) , just scared my self a little. 
[Edited on 24.6.2011 by IPN]
Finished the addition few hours ago, started N2 flow and increased the heating on the MeBr flask for few minutes. Picture of TLC attached (1:5
EtOAc:hexane; product left, vanillin right; developed with 20% H2SO4)
I think I lost some MeBr through leaks as the inlet tube was clogged with KBr.  Might need to run more MeBr through it tomorrow.
Might need to run more MeBr through it tomorrow.
[Edited on 24.6.2011 by IPN]
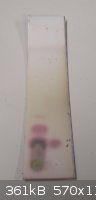
[Edited on 24.6.2011 by IPN]
|
|
|
jzhhua
Harmless

Posts: 2
Registered: 27-6-2011
Member Is Offline
Mood: No Mood
|
|
I think the methyl iodide is an good methyl reagent
|
|
|
| Pages:
1
2 |