| Pages:
1
2
3 |
StevenRS
Hazard to Self
 
Posts: 72
Registered: 31-12-2007
Member Is Offline
Mood: No Mood
|
|
Uranium Isolation
I have recently acquired a Geiger counter, and have found some "hot rocks", some reading over 19,000 CPM. I think they are carnotite, and some have
green crystals with black flecks, possibly uraninite.
It could be and interesting process to purify this ore to actual metal, or even a salt. I was wondering what methods could be used for maximum uranium
extraction, and then a method to extract the uranium salts from the iron and other metal salts sure to be present.
I have heard of the alkaline extraction method, but this produces low yields. The industry uses it because it is cheap.
Also, most uranium salts are soluble, including the carbonate, so maybe converting the mixed salts to carbonate, filtering out the other insoluble
salt out would be possible as a method of purification.
Converting this carbonate (or any other salt) to an oxide would be easy, but converting U<sub>3</sub>O<sub>8</sub> to U could
be difficult.
Any thoughts?
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
http://www.unitednuclear.com/extract.htm
The second page is missing maybe someone could fill it in for him?
[Edited on 18-5-2008 by crazyboy]
|
|
|
StevenRS
Hazard to Self
 
Posts: 72
Registered: 31-12-2007
Member Is Offline
Mood: No Mood
|
|
I have read that page, and it is informative, but the alkaline method produces low yields and with my small supply of ore this is not ideal.
Would a stronger base, KOH maybe, work better?
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
I found it amusing that on the solvent extraction page for uranium they claim that they haven’t been able to update it because the process requires
sulfuric acid--which they apparently 'haven’t gotten in yet.' *raises an eyebrow*
I personally don’t like united nuclear, not just for their prices.
That said, Uranyl Hydroxide does exist, though it is commonly precipitated from oxidized uranium at neutral pH. Certain papers describe the
volatilization of uranium under strongly oxidizing conditions, which may allow its distillation:
http://dx.doi.org/10.1016/j.jnucmat.2005.07.013
Uranyl Chloride may be an option for extraction as well, it forms from uranium dioxide at red heat under chlorine gas, or dissolving uranium oxide in
HCl. It is unstable, and decomposed by light, which may facilitate recovery:
The company Indian Rare Earths Limited (IREL) has developed a process to extract uranium from the Western and Eastern coastal dune sands of India.
After pre-processing with high intensity magnetic separators and fine grinding, the mineral sands (known as monazite), are digested with caustic soda
at about 120C and water. The hydroxide concentrate is further digested with concentrated hydrochloric acid to solubilise all hydroxides to form a feed
solution composed of chlorides of uranium and other rare earth elements including thorium. The solution is subjected to solvent extraction with dual
solvent systems to produce uranyl chloride and thorium oxalate. The crude uranyl chloride solution is subsequently refined to nuclear grade ammonium
diuranate by a purification process involving precipitation and solvent extraction in a nitrate media.
This may be the best solution, as it will also recover other interesting metals and is likely the extraction of choice when you are not positive it is
uranium causing the radioactivity. I should note that Uranyl Chloride is reported as being toxic to the point of humor if ingested.
Another interesting note:
It is precipitated during production by adding aqueous ammonium hydroxide after uranium extraction by tertiary amines in an organic kerosene
solvent. This precipitate is then thickened and centrifuged before being calcined to uranium oxide. Canadian practice favours the production of
uranium oxide from ammonium diuranate, rather than from uranyl nitrate as is the case elsewhere.
There is also, of course, digestion with nitric acid, as the united nuclear site mentions.
Ancillary:
http://en.wikipedia.org/wiki/In-situ_leaching
http://en.wikipedia.org/wiki/Uranium_mining
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Y'think soaking it in molten sodium chlorate would do it?
...Hey, I have a lot of sodium chlorate on hand, okay?... 
Tim
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
Lee, Concise Inorg. Chem., 5th ed, has this to say about industrial U extraction... paraphrasing...
Ore is crushed and concentrated via flotation. Then it is roasted in air, and leached with H2SO4 in the presence of MnO2 to ensure all U goes as
U(VI). This is precipitated as Na diuranate ('yellowcake'). This dissolves in HNO3 as uranyl nitrate, and can be extracted into tributyl phosphate
(c. 20% accd another source) in kerosene.
I wonder how available tributyl phosphate is.
| Quote: | Originally posted by ShadowWarrior4444
I personally don’t like united nuclear, not just for their prices.
|
I wonder why? It is true, the guy behind UN is definitely a kook, he is the one responsible for the alien dissection movie myth and a lot of the Area
51 nonsense. But, they do have some interesting stuff, and not all of their prices are bad, I guess it depends on what. Given the limited number of
hobbyist suppliers, I am willing to tolerate eccentricity.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
It seems like tributyl phosphate would be an ester of butanol and phosphoric acid.
Seems like it wouldn't be exceedingly difficult to make.
Wikipedia has this to say (just remember to take it with a grain of salt):
| Quote: |
Tributyl phosphate is manufactured by esterification of orthophosphoric acid with butyl alcohol. A laboratory synthesis proceeds with phosphorus
oxychloride: [1]
POCl3 + 3 C4H9OH → PO(OC4H9)3 + 3 HCl
http://en.wikipedia.org/wiki/Tributyl_phosphate
|
OrgSyn citation on Wikipedia page:
^ G. R. Dutton and C. R. Noller (1943). "n-Butyl phosphate". Org. Synth.; Coll. Vol. 2: 109.
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv2p0109
That sucks... It would probably be easier to find and buy tributyl phosphate (TBP) than to find or make phosphorus oxychloride. I'm sure there are
other ways, however...
[Edited on 5-18-2008 by MagicJigPipe]
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
| Quote: | Originally posted by pantone159
Lee, Concise Inorg. Chem., 5th ed, has this to say about industrial U extraction... paraphrasing...
Ore is crushed and concentrated via flotation. Then it is roasted in air, and leached with H2SO4 in the presence of MnO2 to ensure all U goes as
U(VI). This is precipitated as Na diuranate ('yellowcake'). This dissolves in HNO3 as uranyl nitrate, |
once you get to this stage, make a ppt with oxalic acid, as uranyl oxalate is not very soluble at all, then heat the oxalate to decomp into uranium
oxide, then use as thermit to form the metal.
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Some older processes, more focussed on radium, would crush and finely grind the ore, air roast it to oxidise sulfides and remove arsenic, then fuse it
with potassium or sodium bisulfate (believe you can use equal molar mix of K/Na sulfate and ammonium sulfate). Crush the cooled melt, extract with
warm dilute H2SO4, filter the extract and add an excess of Na2CO3 to precipitate the iron group, aluminium, any remaining calcium, and a few other
metals. Concentrate, but not so far as to cause crystals or ppt to form, add strong NH3 + NH4Cl to remove vanadium as NH4VO4. Filter, boil the
solution to get a ppt of ammonium diuranate (NH4)2U2O7; igniting this in air at ~500 C gives UO3.
Additional purification would involve reactions as suggested above. Another useful purification step is the precipitation of uranyl peroxide at a pH
of 3-4 using 35 to 50 percent H2O2.
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
Also look into TRU resin it catches 3+ Actinides
of course thorium removal takes HF acid... and they are slow
What would you like to do with your nuclear material?
A nuclear battery wold be just dandy
SO... i really encourage you to remember Paul Brown!!!!!!!
http://users.erols.com/iri/Pauleulogy.htm
http://www.rhfweb.com/paulresnuc.htm
ive read about his invention and i think the concept is quite valid
think nuclear powered tesla coil.
actually
http://www.dow.com/liquidseps/prod/pt_u.htm
these resins are more for Uranium
[Edited on 20-5-2008 by roamingnome]
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Anyone have a proposal for uranyl acetate to uranium metal without ridiculous reducing agents (Li) and dangerous gases?
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Perhaps http://www.sciencemadness.org/lanl1_a/lib-www/la-pubs/003180... could be modified for the acetate?
Of course, one would have to take precautions to avoid uranium vapours from aerosols and non-zero vapour pressures of uranium compounds.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Uranium is difficult to reduce. The lower oxides are rather stable, the element combines with many non- and semi- metals so it tends to form carbides
UC2 mp 2400 C), nitrides (U3N4 decomposes in vac ~1400 C), &ct.
Uranyl acetate decomposes below 300 C, the carbon and oxygen content also make it an unlikely direct precursor.
The methods I've seen listed are basically reduction of U3O8 with Mg in an arc furnace in H2, the action of K, Na, or Na-Mg allow on UCl4 or
UCl4.2NaCl at 700 to 800 C; electrolysis of UCl4 or UCl3 in fused alkali or alkali+alkaline earth chlorides; electrolysis of aqueous solution of UCl4
with a mercury cathode under an atmosphere of hydrogen followed by distillation of the mercury; and the Ames process - effectively a thermit reaction
using calcium or magnesium. All but the fused salts electrolysis and Ames process give a pyrophoric powdered metal.
|
|
|
StevenRS
Hazard to Self
 
Posts: 72
Registered: 31-12-2007
Member Is Offline
Mood: No Mood
|
|
I have heard that uranium oxide(s) can be reduced using aluminum and magnesium at high temperature. Sound fun. I wonder how high of a temp?
Maybe someone could compile a list of common, easily formed, insoluble uranium salts? I am pretty sure it will be a short list...
I think Ammonium uranyl carbonate is insoluble. Could one leach the uranium form an ore with a carbonate/bicarbonate, and then convert the resulting
Uranyl Tricarbonate to the ammonium salt with ammonia gas?
One could convert the tricarbonate to the sulfate, and extract that, as I believe it is also insoluble.
Is the hydroxide soluble in basic solutions? If so, that opens up a possible route of extraction from the ore.
Edit: Found this on wikipedia--
"In the classical procedure for extracting uranium, pitchblende is broken up and mixed with
sulfuric and nitric acids. The uranium dissolves to form uranyl sulfate, and sodium hydroxide is added to make the uranium precipitate as sodium
diuranate, This older method of extracting uranium from its uraninite ores has been replaced in current practice by such procedures as solvent
extraction, ion exchange, and volatility methods."
Seems easy enough. Would the sulfuric acid method work on other ores than urananite?
[Edited on 20-5-2008 by StevenRS]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The reduction of uranium oxides does not appear to be a very desirable route. Historically such efforts tended to give the lower oxides or mixtures
of uranium with oxides, nitrides, or carbides. Such methods also tend to produce the uranium as a pyrophoric finely powdered metal.
They have been done. One early old process was to mix "uranium oxide" with an excess of powdered magnesium, compact that into rods, then place the
rods in a special arc furnace in an atmosphere of hydrogen. After cooling the hydrogen was flushed out with CO2, the finely powdered uranium was then
washed with dilute acetic acid to remove excess magnesium.
If you have carnotite, you have vanadium which tends to follow uranium around to some extent. Removing the vanadium as ammonium metavanadate, or
uranium as peroxide, are two methods of getting good separation, I believe. Thorium and some cerium will go with uranium with the peroxide, but they
are easy to separate at other stages.
Extraction of ores with alkalies tends to drag along silica, which must be separated at a later stage; repeated fuming down with H2SO4, then
dissolving the metal ions out with acid, is a common way of doing that.
Changing the oxidation state of the uranium and precipitating it or impurities, followed by another change in oxidation state to switch solubility,
seemed to be common. A solution of a uranyl salt in water with alcohol or glucose, exposed to sunlight will slowly form an insoluble violet to black
hydrate oxide of mixed oxidation states; air oxidises it to UO3.
| Quote: | | All uranates, even those of the alkalies and ammonium, are insoluble. Ammonia will precipitate from a solution of a uranyl salt the di-uranate
(NH)2[U2O7], a yellow powder practically insoluble in water, but easily soluble in ammonium carbonate solution; this salt can be used to estimate
uranium, but is liable to go over into the colloidal state |
which means it can be major hassles to filter, and not likely to be formed from carbonate complexes.
Uranyl iodate has a low solubility, as does the sulfide and oxalate; the oxalate forms soluble complexes while the sulfide dissolves in dilute acids
and is readily oxidised by air.
The extraction, ion exchange, and volatility methods tend to be industrial type ones, unless you're set up to work with UF6. I think you'll have
better luck looking in old chemistry texts from before WW-II into the Victorian era; they use less exotic reagents and conditions.
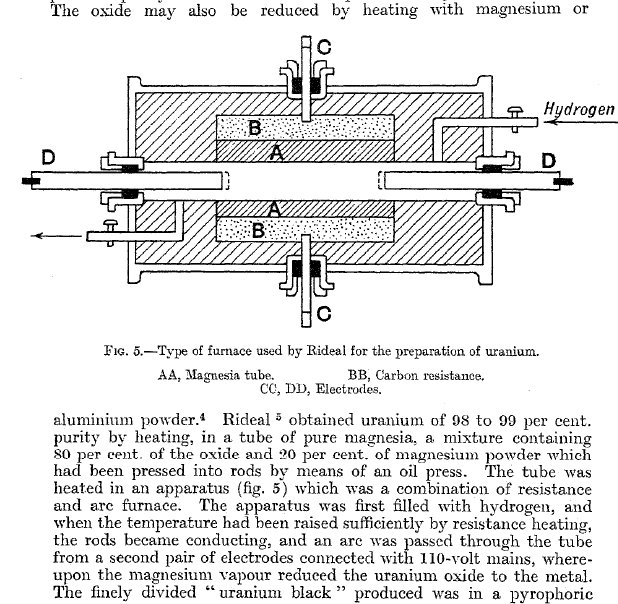
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
btw - Lee also added re U metal prepn:
Final steps are conversion to UF4 followed by reduction with Ca or Mg to yield metal U.
Even if U metal is too hard, just getting any purified U compound from ore would be cool.
|
|
|
StevenRS
Hazard to Self
 
Posts: 72
Registered: 31-12-2007
Member Is Offline
Mood: No Mood
|
|
This might be an easy method of getting pure metal, and it only requires a lightbulb! (kinda)
Place uranium iodide in close contact with a white hot tungsten filament, and the iodide sublimates, contacts the wire, and is decomposed, yielding
very pure metal, minus the tungsten. As the amount of deposited metal increases, the resistance goes down, so more current must be applied.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I knew this was familiar
http://www.sciencemadness.org/talk/viewthread.php?tid=6714&a...
And it appears the van Arkel and de Boer method is a little tricky when used with uranium, the decomposition temperature of the iodides is high enough
that it must be done near the melting point of the metal, so careful temperature control must be done.
http://jphyscol.journaldephysique.org/index.php?option=artic...
van Arkel - de Boer generally uses a small amount if the iodide, and a lot of the free metal in powdered form, in an evacuated envelope along with the
heated filament. The powdered metal is kept hot enough to react with free iodine, which is formed when the iodide decomposes on the filament. If just
the iodide is used, the iodine formed increases the pressure, both conducting heat away from the filament and reversing the decomposition. With some
elements a mixture of a volatile halide and hydrogen is passed over the hot filament in a flow-through system.
And this could be useful in checking solubilities :
http://www.archive.org/details/dictionaryofchem014380mbp
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
| Quote: | Originally posted by StevenRS
It could be and interesting process to purify this ore to actual metal, or even a salt.
I was wondering what methods could be used for maximum uranium extraction.
Any thoughts? |
Here's one!
The most interesting thing about uranium besides its extreme toxicity is its radioactivity.
However, if you can get your hands on enough of the 235 isotope things'll get really really interesting.
And some guy, Osama bin. . .something might just be interested, too.
You could call him. . .
P
|
|
|
StevenRS
Hazard to Self
 
Posts: 72
Registered: 31-12-2007
Member Is Offline
Mood: No Mood
|
|
I finally got my ore ground to powder, I mean dust. The stuff flows like a liquid, three days in a ball mill with 3/4 chrome steel media will do that.
I liked 3 extractiom methods, the peroxide extraction, sodium hydroxide, and sulfuric acid. The peroxide has already reacted a bit, and the solution
is a bit yellow. L
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by Pulverulescent
Here's one!
The most interesting thing about uranium besides its extreme toxicity is its radioactivity.
However, if you can get your hands on enough of the 235 isotope things'll get really really interesting.
And some guy, Osama bin. . .something might just be interested, too.
You could call him. . .
P |
Not all radioactive substances are equally detrimental to life, this is a common misconception. Uranium emits Alpha radiation, which cannot pass
through solid matter nor a few meters of air. Alpha radiation has not ever been known to cause a human cancer, as well. This is likely because it is
in the form of a helium nucleus, whereas the other forms of radiation are subatomic, and therefore more able to penetrate shielding and damage cells.
Uranium can be effectively shielded by a piece of paper.
And the term "extreme toxicity" I reserve for things like Cyanogen Bromide, Dimethyl Mercury, and on a bad day Nickel Carbonyl.
Osama would not know what to do with raw 235; now Iran, the ayatollahs would pay quite a sum for the services of an enrichment expert. (As they have
for Russian ex-patriots with *any* knowledge of nuclear physics.)
| Quote: | | I finally got my ore ground to powder, I mean dust. The stuff flows like a liquid, three days in a ball mill with 3/4 chrome steel media will do that.
I liked 3 extraction methods, the peroxide extraction, sodium hydroxide, and sulfuric acid. The peroxide has already reacted a bit, and the solution
is a bit yellow. L |
I'm quite interested in seeing how the peroxide extraction turns out, as of the three you've chosen, it is the only one that is not used appreciably
on an industrial level. Can you photograph each stage of the process? [This might make a good addition to the prepublication section--perhaps a
treatise on OTC ore extraction processes.]
[Edited on 5-21-2008 by ShadowWarrior4444]
|
|
|
StevenRS
Hazard to Self
 
Posts: 72
Registered: 31-12-2007
Member Is Offline
Mood: No Mood
|
|
I had to go out of town, so my test samples are 500 miles away, butt I will definatly take pictures when I return.
What else would the peroxide method extract, other than cerium or thorium?
I wonder what could be used to precipitate the uranium out of this solution?
Maybe conversion to the sulfate? Or the carbonate, followed by neutralization?
I just read about a very efficent extraction process using sulfuric acid and an oxidiser, sodium chlorate. Maybe H2O2 could be substituted?
"To produce this form of yellowcake (magnesium diuranate), crushed ore is mixed with hot water to a 58% solids slurry. The solids slurry is then
processed through a series of tanks, where sulfuric acid, sodium chlorate, and steam are used to extract the uranium from the solids slurry. The
average leaching efficiency for this process is 98.5%. The uranium-bearing solution is then decanted and directed to a solvent extraction (SX) process
for further purification. In this extraction step, the dissolved uranium is transferred from the feed solution into the organic solvent. Next a
stripping step recovers the uranium into a sodium chloride aqueous phase after which the barren solvent is recycled. The average efficiency of the SX
circuit is 99.9%. The high-grade “pregnant” strip solution from SX goes to the next stage where magnesia slurry is added to precipitate magnesium
diuranate."
It does sound a bit compicated, though. I would prefer something that did not use an organic solvent extraction phase, and is less steps.
[Edited on 21-5-2008 by StevenRS]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
| Quote: | Originally posted by ShadowWarrior4444
Not all radioactive substances are equally detrimental to life, this is a common misconception. Uranium emits Alpha radiation, which cannot pass
through solid matter nor a few meters of air. Alpha radiation has not ever been known to cause a human cancer, as well. This is likely because it is
in the form of a helium nucleus, whereas the other forms of radiation are subatomic, and therefore more able to penetrate shielding and damage cells.
|
Uranium is relatively harmless because it has such a long half-life. Alpha emitters are the least dangerous materials externally but among the most
damaging inside the body. The relatively high mass and charge of an alpha particle leads to high linear energy transfer which in turn leads to
concentrated damage on a cellular level. The famous alpha emitter radium contributed to many human cancers before its properties were well-understood.
The Russian dissident Alexander Litvinenko was killed in a high profile incident only 2 years ago with the alpha emitter Polonium-210. Alpha emitters
as a group are certainly not a safer category of radioactive materials.
PGP Key and corresponding e-mail address
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by ShadowWarrior4444
Alpha radiation has not ever been known to cause a human cancer, as well. This is likely because it is in the form of a helium nucleus, whereas the
other forms of radiation are subatomic, and therefore more able to penetrate shielding and damage cells. Uranium can be effectively shielded by a
piece of paper.
And the term "extreme toxicity" I reserve for things like Cyanogen Bromide, Dimethyl Mercury, and on a bad day Nickel Carbonyl.
|
Your first statement is erroneous. Alpha emitters pose little harm while outside the body, however inside the body they are extremely dangerous as
they emit right next to your cells, damaging them. Hence any alpha emitter becomes dangerous, especially in powder form, and alpha emitting dusts
are known to cause cancer.
While the ore grindings may not be too dangerous in the low doses expected with grinding down to a fine powder, respiratory protection would be
strongly advisable.
And defiantly in any later steps with purified uranium compounds, all precautions must be taken to avoid inhalation.
I would sooner work with cyanogen bromide than radioactive dusts.
EDIT: Looks like Polv' beat me to it by 5 min
[Edited on 21-5-2008 by The_Davster]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by StevenRS
I wonder what could be used to precipitate the uranium out of this solution?
Maybe conversion to the sulfate? Or the carbonate, followed by neutralization?
I just read about a very efficent extraction process using sulfuric acid and an oxidiser, sodium chlorate. Maybe H2O2 could be substituted?
... |
We've been listing those sorts of things. The sulfate, unlike simple sulfates such as those of lead or barium, is a basic sulfate UOSO4 . 2H2O formed
under proper conditions of pH and concentration. The simple sulfates, such as U(SO4)2 . n H2O or UO2SO4 . n H2O, are soluble in water.
The Radiochemistry of Uranium
http://library.lanl.gov/cgi-bin/getfile?rc000030.pdf
http://www.sciencemadness.org/lanl1_a/lib-www/books/rc000068...
has some useful information:
| Quote: | Peruranates are formed when uranyl solutions containing hydrogen peroxide are made alkaline. The composition of the peruranates depends upon the
concentration of the alkali and peroxide. The followlng groupO have been identified: M2U2O10 . XH20, M2U06 . XH20, M6U2013 . XH20, and M4U08 . XH20.
The peruranates are generally soluble In water. The least soluble are those of the M2U2010 . XH20 group. The peruranates are soluble In dilute mineral
acid.
|
and
| Quote: | Hydrogen peroxide precipitates uranium
peroxide, U04 . XH20, from sllghtly acldlc solutions. The
reaction occurs In the pH range 0.5-3.5. The optimum range
IS 2.0-2.5. Hydrogen Ions released with the formation of
uranlvm peroxide are neutralized with ammonia or ammonium
acetate. Complete preclpltiatlon requires an excess of
hydrogen peroxide. Quantltatilve separation may be effected
by freezing the aolutlon, allowing It to &tend, and filtering
at 2°C. The separation from most elements Is good since It
Is done from an acidic solution. plutonium, thorium,
hafnium, zlrconlum, and vanadium aleo precipitate. Iron
interferes by catalytically decomposing hydrogen peroxide.
Small quantities of Iron may be complexed with acetic, lactlc,
or malonlc acid. Low yields may result from the use of
malonic. acid. Ammonlum, potaaslum, and alkaline earths retard
the rate of precipitation. Complexlng Ions such as
oxalate, tartrate, sulfate, and fluoride In large quant%tiea,
also Interfere. Fluoride Ion may be complexed with alumi-
-.- |
Th e extraction processes generally use counterflow extraction, doing them in a home lab means doing a series of extractions and back extractions,
often not practical except for those with OCD.
One extractive method that might be practical would be to convert to crude uranyl nitrate, add a small amount of aluminium sulfate to tie up any
fluoride, evaporate to dryness, and extract with acetone, isopropyl or ethyl alcohol, then evaporate off the solvent to obtain the purified uranyl
nitrate.
|
|
|
| Pages:
1
2
3 |