Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
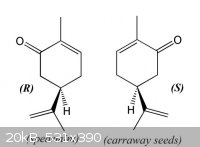
The smell of chirality: carvone enantiomers
(R)-(-)- & (S)-(+)-Carvone are examples of how the same molecule can smell very differently depending on its chirality &
consequent interaction with olfactory receptors in the nose.
The diffrerent enantiomers of carvone are biologically active:
| Quote: | Chirality. 2007 May 5;19(4):264-8.
Influence of the chirality of (R)-(-)- and (S)-(+)-carvone in the central nervous system: a comparative study.
de Sousa DP, de Farias Nóbrega FF, de Almeida RN.
Departamento de Fisiologia, Universidade Federal de Sergipe, CEP 49100-000 São Cristóvão, Sergipe, Brazil.
Many terpenes are used therapeutically, and as flavor and fragrance materials. (R)-(-)-Carvone, the main constituent of spearmint oil, and
(S)-(+)-carvone, found as major component of caraway and dill seed oils, have several applications and are used in cosmetic, food, and pharmaceutical
preparations. In this study, the effect of enantiomers of carvone on the central nervous system (CNS) was evaluated in mice. The LD50 value was 484.2
mg/kg (358.9-653.2) for (S)-(+)-carvone, and 426.6 (389.0-478.6) mg/kg for (R)-(-)-carvone. Both enantiomers caused depressant effects, such as
decrease in the response to the touch and ambulation, increase in sedation, palpebral ptosis, and antinociceptive effects. (S)-(+)- and
(R)-(-)-carvone caused a significant decrease in ambulation. (R)-(-)-Carvone appeared to be more effective than its corresponding enantiomer at 0.5
and 2.0 h after administration. However, (S)-(+)-carvone was slightly more potent at 1 h. In potentiating pentobarbital sleeping time, (R)-(-)-carvone
was more effective than (S)-(+)-carvone at 100 mg/kg, but was less potent at 200 mg/kg compared to the (+)-enantiomer, indicating a sedative action.
(S)-(+)-Carvone at the dose of 200 mg/kg increased significantly the latency of convulsions induced by PTZ and PIC, but (R)-(-)-carvone was not
effective against these convulsions. These results suggest that (S)-(+)-carvone and (R)-(-)-carvone have depressant effect in the CNS. (S)-(+)-Carvone
appears to have anticonvulsant-like activity.
|
Reference on the extraction of carvone enantiomers from spearmint leaves & caraway seed: http://cnx.org/content/m15591/latest/
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Caraway Oil Extraction (Carvone)
I always find extracting things to be a fun way to pass the time and the result is usually rewarding, albeit the yields are usually very low compared
to the effort input.
Carvone is interesting because as mentioned above, the R -(-)- isomer is found is many species of mint (particularly spearmint), giving it the
characteristic mint essence. The S -(+)- isomer on the other hand is what gives caraway it's characteristic scent. The oils extracted from both
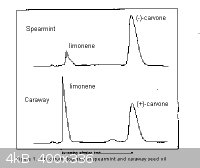
spearmint and caraway also contain various other compounds, mainly limonene. Caraway oil contains a higher proportion of limonene than does spearmint
oil, and spearmint oil contains a higher amount of R-carvone than carway does of the S isomer.
Anyhow, this is how the extraction was done. It's a typical steam distillation with solvent extraction.
26 grams of dried caraway- Interestingly enough caraway is not a seed, rather these are properly referred to as caraway fruits. They were
ground for a few minutes in a small kitchen food processor, creating lots of powerful smelling dust that went everywhere.This was then loaded into a
500ml RBF along with 150 ml of water
 
Lacking a heating mantle an aluminum foil skirt was added to improve heating over the hotplate. 50ml of fresh water was added to the pot for every 50
ml of distillate collected, until 200ml was obtained. It took about an hour, here it is sitting in the fridge, oily drops can be seen floating on the
surface.
 
Next was solvent extraction. First the distillate was salted with NaCl, and then extracted with two 30ml portions of DCM. The lower phase contains the
caraway oil. The solvent was then dried over sodium sulfate for an hour, with the occasional shaking.
 
Next step is to remove the DCM from the oil via distillation. A hot (but not boiling) water bath was used for this as DCM boils at just under 40c. The
solvent was collected in an ice bath for recycling.
 
The result after all of the DCM was distilled off was the following; 0.73g of oil (50-75% R-(+)- carvone). It has the original smell of caraway,
except much stronger and perhaps muskier? It has a scent reminiscent of damp hay or wood.... and depending on how much you smell it, it starts to
smell like spearmint that has went stale. Is this because some of the olfactory nerves are incorrectly interpreting it as the S -(-)- isomer? Anyways,
this was a fun way to kill some time!
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Cont...
The last two pictures didn't upload on the previous post, here is the final product:
 
|
|
|
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
Very nice extraction! I'll have to repeat this procedure...
Fear is what you get when caution wasn't enough.
|
|
|
|