| Pages:
1
2 |
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
Syntheses of 3-quinuclidinol
There are several published syntheses of 3-quinuclidinol. This one (US3464997) caught my eye because it was developed by the U.S. Army at Edgewood
Arsenal.
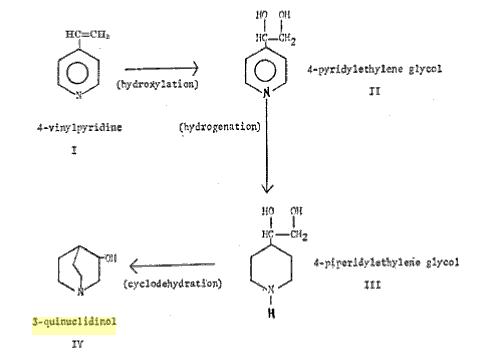
The attached screenshot of the patent process flow diagram summarizes this method:
1. 4-Vinylpyridine (a commercially available derivative of gamma-picoline) is converted to the derivative glycol with permanganate;
2. The pyridyl glycol is hydrogenated over PtO2 to give the 4-piperidinyl glycol;
3. This glycol is cyclodehydrated at 300 deg over basic alumina in the gas phase to yield 80% 3-quinuclidinol & isomeric products.
Apparently the U.S. Army was really ready to play hard with BZ!
[Edited on 15-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Um, there may be bits missing.
From III to IV you perform a cyclodehydration but you introduce a 3 hydroxyl substituent at the same time, pretty amazing chemistry if it works!
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by ScienceSquirrel
Um, there may be bits missing.
From III to IV you perform a cyclodehydration but you introduce a 3 hydroxyl substituent at the same time, pretty amazing chemistry if it works!
|
Only the terminal hydroxyl cyclodehydrates. See the attached graphic. Have you read the patent? That scheme is right from it.
[Edited on 16-7-2008 by Ritter]
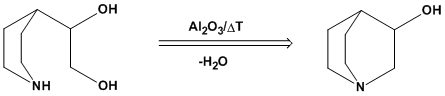
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Nice. I had not seen that one before.
4-vinylpyridine is useful. I use it to prepare polyvinylpyridine chlorochromate resin beads, crosslinked with divinylbenzene. Very effective
solid-supported oxidizing agent for primary alcohols to aldehydes. aka PVPCC.
4-vinylpyridine is a monomer. You have to keep it cold and in the dark or it tends to polymerize, in which case it needs to be distilled.
I took a look at this patent. As a preliminary observation, this does not look at all like a finished industrial process, but rather a bench scale
one. The usual statement is made about the shortcomings of the prior art, such as low yields, complex reactions etc. but contrary to the claims I do
not see a vast improvement here. The first step is a KMnO4 oxidation, just as in the classical route, from 4-methylpyridine itself rather than from
4-vinylpyridine. Since 4-vinylpyridine is made from 4-methylpyridine via isonicotinic acid its oxidation process, then this process shares a common
starting point with thatone.
Of course in both cases the processes can be truncated by starting with the commercially available intermediates. Isonipecotic acid is commercially
available so the oxidation of 4-picoline and hydrogenation steps can be skipped.
Low yields? The yield from the permanganate oxidation of 4-vinylpyridine is unimpressive.
The catalytic hydrogenation of the pyridine in the patent is done under mild conditions (60 psi in a Parr shaker at ambient over PtO2). That of the
N-alkylated ester of isonicotinic acid, is done at 100 psi and 100 C over Pd/C, which is a little high for a Parr bottle but not impossible. The
Org.Syn. article says 1000 psi but then comments in a footnote that 100 psi did just as well.
The classical cyclization uses potassium, but it is a good bet the cheaper and less hazardous sodium works as well.
The patent cyclicization in vapor phase over basic alumina is not very attractive or efficient as two side products are formed. Id look for
alternatives and improvements in this step. The reaction appears to depend on a highly specific brand of alumina, that being Woelm basic alumina from
Alupharm Chemicals, New Orkeans, Louisiana. I happen to be from New Orleans and never heard of this company, my first guess would be that very likely
posy-Katrina they are no longer there. There is zero Internet presence, but the parent company appears to be French, they show up in some business to
business sites in French but do not seem to have any website of their own.
Id be curious about what process was actually used. BZ was manufactured, weaponized and stockpiled, and just because this patent was by EA does not
mean it was the one selected for scaleup.
[Edited on 16-7-2008 by Sauron]
[Edited on 17-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The patent:
Step 1. Oxidation. 35% yiels
Step 2: Conversion to hydrochloride salt 51% yield
Step 3: Hydrogenation 64% yield
Step 4: Cyclization: 32% recovered yield.
GC reported 80% of the product was 3-quinuclidinol but only 32% could be isolated from the byproducts.
Overall yield c.4%.
Here's the patent document:
[Edited on 16-7-2008 by Sauron]
Attachment: US3464997.pdf (134kB)
This file has been downloaded 488 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Now let's look at the Org.Syn. procedure (for the 3-quinuclidone).
Seps 1 and 2 Starting from ethyl isonicotinate, N-alkylated with ethyl bromoacetate, 64-78% comb
Step 3: Dieckmann cyclization 77-82% overall yield
Overall 49-64% for the three steps
Of course we still must reduce this, usually with LAH, but that's hardly terra incognita and the tield ought to be very good.
Here's the Org.Syn. article, with references and notes, see for yourself.
I do not see any great difficulties in this prep. Ethyl bromoacetate is nasty, but that's what fume hoods are for. Potassium metal can probably be
replaced by either sodium metal or preformed, freshly opened potassium ethoxide, commercially available. Ethyl isonicotinate is just as commercially
available as 4-vinylpyridine. The over all yield is MUCH better. And the number of steps is comparable.
[Edited on 16-7-2008 by Sauron]
Attachment: CV5P0989.pdf (141kB)
This file has been downloaded 516 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I see what you are doing now.
Personally I think the Org Syn method is better.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I think there may be room for a lot of improvement in that Edgewood patent. There certainly ought to be a batter reagent than permanganate for
hydrating a pi bond (conjugated and terminal) to glycol and the final step, surely can be managed better?
I am looking up one such possibility now.
Since the nuclear hydrogenation yield isn't bad, if the yields in the first and last steps can be improved then this process may be more viable.
A paper in J.Org.Chem describing a new route to quinuclidine is cited in the patent. There is also a J Org Chem paper by the inventors, in which they
credit the earlier work as inspiring them to try the same route to 3-quinuclinol.
I have obtained the earlier paper. In it, 4(2-hydroxyethyl)pyridine is prepared by reaction of gamma picoline and formaldehyde. This alcohol,
isoolated from other products of the reaction, is reduced, distilled through a column of hot alumina and cyclized to 1-azabicyclo[2.2.2]octane but the
yield is poor From 40 g of 4-(2-hydroxyethyl)piperidine only 11 g isolated yield is obtained.
The inventors at Edgewood reasoned that a piperidyl glycol could be made to undergo the same reaction. They were correct, but the yield was no better
than with the piperidylethanol, and 20% of the distillate also hydrolyzed to the alcohol during hydrogenation. The seperation of the alcohol from the
glycol was costly of product.
[Edited on 16-7-2008 by Sauron]
Attachment: jo01059a502.pdf (275kB)
This file has been downloaded 598 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I have gotten the JOC peper corresponging to the Edgewood patent. As expected it is somewhat more detailed than the patent examples.
The alumina use, Woelm neutral or preferably basoc alumina, from Alupharm Chemicals, was the only one of five commercially available aluminas tested
that produced a "reasonable" yield, that being 32% only.
I do not know if this brand is still available but will check. If not then one would be forced into an easter-egg hunt for a replacement alumina. That
would not be at all encouraging.
Runs with calcium, strontium and barium oxides produced results inferior to alumina.
Some suggestions were made to potential improvements in the hydrogenation step.
See attached article.
[
[Edited on 17-7-2008 by Sauron]
Attachment: jo01015a561.pdf (482kB)
This file has been downloaded 653 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
I think there may be room for a lot of improvement in that Edgewood patent. There certainly ought to be a batter reagent than permanganate for
hydrating a pi bond (conjugated and terminal) to glycol and the final step, surely can be managed better?
I am looking up one such possibility now.
|
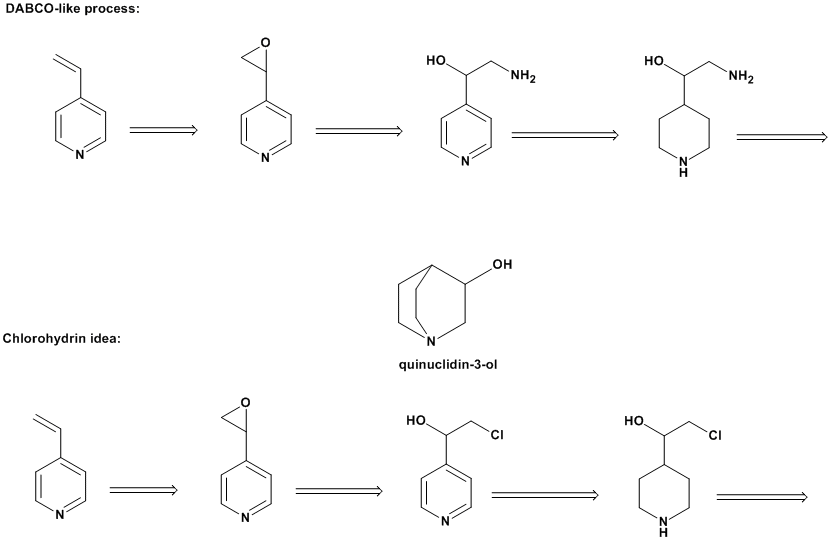
You have the option of epoxidizing the 4-vinylpyridine, then hydrolyzing that to the glycol. Or reacting the epoxide with ammonia to form the
alkanolamine or with HX to get the halohydrin.
To obtain the halohydrin directly from 4-vinylpyridine, I believe the addition of HOX is a standard method.
DABCO, a urethane catalyst, is the aza analog of 3-quinuclidinol. The ChemDraw attachment shows a synthesis scheme based on an early DABCO synthesis.
In this case the leaving group is ammonia. An alternative might involve the other scheme, which cyclizes the chlorohydrin.
I'm also curious as to the workability of esterifying the terminal hydroxy of the(4-piperidyl)-1,2-ethanediol to have a better leaving group for the
cyclization reaction.
[Edited on 16-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
I recall that the pyridine ring can be reduced under mild conditions if it is first quaternized. In this case I would suggest quaternizing with benzyl
chloride so that the N-benzyl group would also be removed as toluene in the same reductive step.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
It would be useful to end up with 3-chloroquinuclidine but for that the hydroxyl needs to be on the beta C and the Cl on the alpha C. That way the
-H2O can occur and Cl remain.
Quinuclidine, 3-quinuclidone and 3-chloroquinuclidine are not CWC scheduled compound and thus anyone can experiment with them. 3-quinuclidinol is
another matter. In most countries which have aceeded to CWC, it is a serious crime to produce or possess CWC scheduled compounds without special
permission. This is a matter of national laws, rather than international accords.
The Australian Group if it ever gets its way would expand CWC coverage to 3-quinuclidone.
Sic gorgeamus a los subjectatus nunc.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Sigma Aldrich seem quite happy to sell 3-quinuclidinol to me.
Mind you, they are only offering 1 or 10g :-D
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Perhaps. Depends on who you are and where you are. Some issues may ensue.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by ScienceSquirrel
Sigma Aldrich seem quite happy to sell 3-quinuclidinol to me.
Mind you, they are only offering 1 or 10g :-D |
To my knowledge you have to have an account with Aldrich in order to buy anything from them and in order to get an account you have to send them
copies of your incorporation documents so they can establish that you are a bona fide academic or commercial organization. And even if you
had an account, buying any quantity of a CW agent precursor would likely get you a visit from the FBI or your nation's equivalent
agency.
[Edited on 16-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I have an account with the S-A and Fluka agent here, and have bought from them, but an order for this compound would require a special import permit
from the Ministry of Defense and would likely not be forthcoming.
The powers that be are really quite afraid of these various toys they themselves created, and do not want anyone dabbling in them.
The 3-hydroxy-N-methyl and N-ethylpiperidines were already covered by DEA schedules and I think also by the UN drug control regimes and related
international conventions long before CWC.
[Edited on 17-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
I have gotten the JOC peper corresponging to the Edgewood patent. As expected it is somewhat more detailed than the patent examples.
The alumina use, Woelin neutral or preferably basoc alumina, from Alupharm Compaigne, was the only one of five commercially available aluminas tested
that produced a reasonable yield, that being 32% only.
|
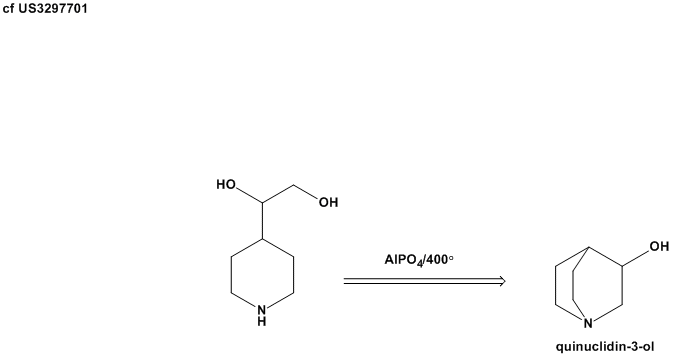
The original DABCO patent states an example where N-(beta-hydroxyethyl)piperazine is converted into DABCO by passing the vapor over aluminum phosphate
at 400 deg C with 50% yield. Aluminum phosphate dissociates to aluminum oxide at high temperatures.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Woelm basic alumina sold by Alupharm - French company, US distributor is or more likely was in New Orleans. I do not know their present status.
Anyone ever heard of these folks?
I originally misread Woelm as Woelin, but Woelm appears to be correct.
I can't see why the conversion of the pyridine glycol to its hydrochloride salt proceeds is 54% yield only. Coupled with the 35% yield in the
oxidation, this really cripples the overall yield to 18% after just two steps. If the aluminum phospate final step proceeds in 50%, then the final
overall yield from 4 steps is still 6% and that frankly sucks.
Tweaking catalytic activity around as suggested to reduce the hydrolyzed byproduct in the hydrogenation, isn't going to improve that very much. 7%? It
looks like a lot of effort would nbe needed to get this to overall 10%.
[Edited on 17-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I have no intention of buying 3-quinuclidinol. It would be a stupid and strange purchase for me.
We don't have have an FBI around here. The USA seems weird to us Europeans, unlimited access to guns but you cannot buy sodium borohydride.
I can buy most chemicals. I have academic qualifications and legitimate reasons for owning them so gaining an account with a chemical supplier is not
a problem.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
| Quote: | Originally posted by Sauron
I have an account with the S-A and Fluka agent here, and have bought from them, but an order for this compound would require a special import permit
from the Ministry of Defense and would likely not be forthcoming.
The powers that be are really quite afraid of these various toys they themselves created, and do not want anyone dabbling in them.
The 3-hydroxy-N-methyl and N-ethylpiperidines were already covered by DEA schedules and I think also by the UN drug control regimes and related
international conventions long before CWC.
[Edited on 17-7-2008 by Sauron] |
And I have to deal with local customs but they are nowhere near as bad as the dipsticks that occupy similar positions in the Far East.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I think Ritter assumed you live in USA, not the case, clearly.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
A word to the wise for those contemplating experimenting with ant-cholingergic agents:
| Quote: | Anticholinergic Drugs May Increase Cognitive Decline
Susan Jeffrey
April 18, 2008 (Chicago, Illinois) — A new study finds that the use of drugs with anticholinergic activity is associated with a more rapid decline
in cognitive performance in older individuals.
The findings suggest that physicians should take this possible effect into account when prescribing anticholinergic medication or drugs that have
anticholinergic properties, said lead author Jack Tsao, MD, DPhil, associate professor of neurology at Uniformed Services University, in Bethesda,
Maryland.
Dr. Tsao presented the results here at the American Academy of Neurology 60th Annual Meeting.
Anticholinergic Properties
Their study arose from experience with a patient seen by study coauthor Kenneth Heilman, MD, from the University of Florida at Gainesville, Dr. Tsao
told Medscape Neurology & Neurosurgery. The woman had come in with memory complaints and hallucinations. However, Dr. Tsao said, "Her cognitive
testing was essentially normal except for the memory issues, so she didn't fit the diagnosis of Alzheimer's-type dementia."
This patient had just begun treatment with tolterodine (Detrol, Pfizer) a drug to treat overactive-bladder problems. "Because Dr. Heilman had seen a
previous case of another woman who had memory complaints that reversed after stopping her bladder medicine, we did the same for this lady, and her
memory improved," he said. "This prompted us to ask the question of whether anticholinergic medicines or medicines that have anticholinergic
properties actually can impair thinking in normal individuals."
Anticholinergic drugs range from the overactive-bladder agents and the Parkinson's-disease agents that are known to be strongly anticholinergic, to
drugs such as warfarin, furosemide (Lasix, Sanofi-Aventis), hydrochlorothiazide, and ranitidine (Zantac, GlaxoSmithKline), an antireflux drug, that
have weaker anticholinergic properties, Dr. Tsao said.
"When we actually looked through the literature, a lot of medicines that are not advertised as anticholinergic in nature actually have anticholinergic
properties in vitro," he said.
To look more closely at this potential problem in a wider population, they used data from the Rush Religious Orders Study, a longitudinal cohort study
enrolling older Catholic nuns and clergy without known dementia at enrollment. Of these, 870 had at least 1 follow-up evaluation.
During baseline and annual clinical evaluations, medication and supplement use was determined by direct observation of pill bottles, and global
cognitive function was measured using a 21-item neuropsychological battery.
They divided subjects into those who had taken any of the drugs on the list they had compiled of those with anticholinergic properties (n = 679);
subjects who had never taken any of these agents (n = 191) were used as the reference group.
The subjects were observed for a mean of 7.8 person-years, the authors note. In piecewise-regression models adjusted for age, sex, and education, the
level and annual rate of cognitive decline for individuals before and after starting an anticholinergic drug were compared with the intercept and
slope for the reference group.
Dr. Tsao and colleagues report that the level and annual rate of change were not significantly different between the medication and reference groups
prior to the use of an anticholinergic agent. However, compared with the reference group, the rate of cognitive decline after anticholinergic use was
0.045 units/year more rapid (P = .0044), the authors write.
"As all subjects were cognitively normal at the time of entry into the Religious Order Study and none were taking medications with anticholinergic
effects, we conclude that initiation of medications with anticholinergic activity is associated with a more rapid decline in cognitive performance,"
the authors conclude.
In future work, they plan to stratify these agents on the basis of the relative potency of their anticholinergic effects to see whether more potent
drugs may have a greater effect on memory compared with those that are less potent, Dr. Tsao concluded. "We're also going to try to see whether those
subjects who might have mild cognitive impairment fare worse."
The study was supported by a grant from the American Philosophical Society and the National Institute on Aging. Dr. Tsao reports that he has received
personal compensation for activities with CME LLC as an independent reviewer; holds stock and/or stock options in Amgen; and has received research
support from Defense Spinal Cord and Column Injury Program, Uniformed Services University, and the Comprehensive Neuroscience Program. Disclosures for
coauthors appear in the abstract.
American Academy of Neurology 60th Annual Meeting: Abstract S51.001. Presented April 17, 2008.
|
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
My apologies for being snappish.
Regulations on the sale of chemicals seem to vary by huge amounts from one country to another.
I live in part of the UK and I can easily purchase reasonable quantities of iodine and THF for example. All that is needed is a credit card number and
some reasonable proofs of identity etc at most.
A certain degree of care is required when purchasing chemicals. Eg buy reasonable amounts for private use, buying huge amounts of 35% hydrogen
peroxide may get you noticed, similarly buying potassium chlorate and sulphur at the same time is not a brilliant idea.
Chemical companys want to sell chemicals to legitimate users, at least around here. However they also want to protect themselves from the trouble
that could be caused by supplying chemicals to people who may have severe accidents or commit criminal offences.
Anyway that is the state of affairs around here at the moment.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by ScienceSquirrel
My apologies for being snappish.
Regulations on the sale of chemicals seem to vary by huge amounts from one country to another.
I live in part of the UK and I can easily purchase reasonable quantities of iodine and THF for example. All that is needed is a credit card number and
some reasonable proofs of identity etc at most.
A certain degree of care is required when purchasing chemicals. Eg buy reasonable amounts for private use, buying huge amounts of 35% hydrogen
peroxide may get you noticed, similarly buying potassium chlorate and sulphur at the same time is not a brilliant idea.
Chemical companys want to sell chemicals to legitimate users, at least around here. However they also want to protect themselves from the trouble
that could be caused by supplying chemicals to people who may have severe accidents or commit criminal offences.
Anyway that is the state of affairs around here at the moment. |
The U.K. also has some interesting drug laws. You can obtain codeine & codeine formulations without a prescription but they limit the number of
bottles you can buy. I was buying codeine with aspirin in a national chemist chain shop in Manchester & they would only sell me (If IIRC) 2
bottles. The manager asked me why I wanted to buy more & I answered that codeine is only available by prescription in the U.S. I asked him why
their were restrictions in the U.K. & he told me that people were choosing codeine to commit suicide.
Codeine has an LD50 of 800 mg for the average person. With a maximum of 8 mg per pill, you would need to consume 100 tablets or more, a level where
the aspirin or acetaminophen would likely kill you first.
http://leda.lycaeum.org/Documents/Codeine_FAQ.11309.shtml
[Edited on 17-7-2008 by Ritter]
[Edited on 17-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
There are plenty of similar examples.
One of the things that I do is make bath fizzies with children.
A bath fizzy, for the uninitiated, is a cocktail of sodium bicarbonate, citric acid etc with some oils and perfume.
Children love making them and they like the finished product as do parents etc. Fill the bath, drop in the bath fizzy and relax 
When I first started on the bath fizzy business I naively went down to my local friendly phamarcist who has been useful in the past to enquire about
obtaining citric acid. He was less than helpful as he could only sell it in 25g packets because people are using it to cook their heroin.
So I found a supplier that sells by the multi kilogram load and I resell to winemaking friends etc.
One of the interesting things about codeine is that it can be reacted with pyridine hydrochloride to form morphine, diacetylation then yields heroin.
It is not happening locally but it seems to a problem elsewhere.
http://www.erowid.org/archive/rhodium/chemistry/codeine.home...
|
|
|
| Pages:
1
2 |