Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
If water absorbs infrared, how come pools don't get hotter?
Discussing with friends on how to heat a swimming pool in winter using the sun, someone suggested to paint the bottom black. It makes sense to me, I
would imagine it would heat faster with a black botton (ugly as hell though) but then I recall that water absorbs IR, so it should make no difference
if the bottom of a pool is black or not.
Swimming pools and pounds should be the perfect solar panels: Since water is not totally opaque to IR, it should penetrate the surface and be absorbed
somewhat deeper.
Anyone has an explanation about why a glass/metal solar panel gets so much hotter than a water pond?
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Perhaps simply the fact that glass and metal have lower heat capacities than water? 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
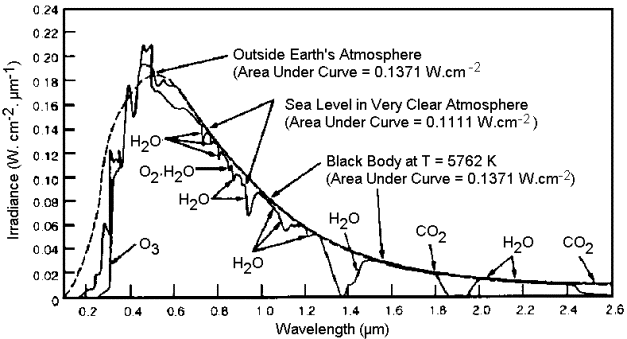
Sunlight approximates the radiation of a black body radiator of around 5780 K, with its peak in the visible range. Much of the energy of such thermal
radiation is in the visible portion of the spectrum; the percentages for UV, visible, and IR, are about 3, 44, and 53. So roughly half the radiation
is in the visible; additionally water does not absorb all IR but mainly in certain bands.
So if you want to get the maximum energy collection you need a good black surface to absorb as much of the spectrum you can. The you need to prevent
your collector from losing energy through heating it environment, such as the air touching it, thus the glass panel and insulation on the back of the
absorber.
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
Evaporation carries a lot of heat away. In case of water this often results in temperature drop of several degrees compored to closed container. If
there is strong wind and dry air around, temperature can drop even more. So one might want to use polymer foil to cover surface of pool to limit
evaporation.
[Edited on 31-7-2008 by chromium]
When all think alike, then no one is thinking. - Walter Lippmann
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Perhaps sheer size and heat capacities? A solar panel is of limited mass compared to a swimming pool, without even taking into consideration the heat
capacities.
Water can get very hot just from the sun, ever leave a waterbottle outdoors only to find it at 50-60C the next time you try to drink from it?
Or solar showers for camping which use a transparent plastic bag left in the sun all day which do get too hot at times.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Make a large teabag from a pillowcase tied with some strong twine and toss it into the pool to make a large suntea brew of the entire swimming pool.
This will offer several advantages, inadvertantly swallowed water will taste better, teastained swimmers will have a simulated tan while also getting
a full body skin absorption dose of caffeine. The tint will also mask any urine which may have been thoughtlessly released into the pool.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
I would bet putting a black tarp over it would make a huge difference. This would help prevent, or at least slow the evaporation, as well as absorb
more heat.
If you think about it, with all the waves, large bodies of water actually have a HUGE surface area.
| Quote: | Originally posted by Rosco Bodine
Make a large teabag from a pillowcase tied with some strong twine and toss it into the pool to make a large suntea brew of the entire swimming pool.
This will offer several advantages, inadvertantly swallowed water will taste better, teastained swimmers will have a simulated tan while also getting
a full body skin absorption dose of caffeine. The tint will also mask any urine which may have been thoughtlessly released into the pool.
|
Or would the tannins, polyphenols and other phytoalexins in the tea change colors from the pH change caused by urine? If so, even better!
|
|
|
Twospoons
International Hazard
    
Posts: 1281
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Water only absorbs IR in narrow bands - the total energy in these narrow bands is not much compared to the overall solar light flux. Hence the use of
broad spectrum absorbers in solar heating.
I like your idea, rosco, but I prefer coffee 
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Uhmm... Only certain bands of IR are absorbed. I think that would be the best explanation. I rule out size, mass and heat capacity because you CAN
heat a pool using a few solar panels, meaning that there is a huge eficiency differerence between both.
Coffe or tea? No. It is hard enough to keep a pool free of algae and bacteria without giving them nutrients. On the other hand, one could mix powdered
activated carbon with the water and then turn on the filters one or two hours before using the pool. Would even remove pollutants.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Certain IR bands plus half the sunlight energy being in the non-IR. You can see the absorption bands for water vapour in the chart I posted to get an
idea how little of the main portion of sunlight is absorbed by water.
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
I’m not sure what part of Brasil you hie from, Tacho, since it’s a huge country from the equator to 40deg S, but surely swimming pools get plenty
hot from sunshine there, at least in summer? They certainly do here in far south US. I find that the solar panels many use (which work well in winter)
make the water unpleasantly warm in summer. Most pools are screened, too. This attenuates the incoming radiation by about 25%, I estimate.
As has been said above, a shallow pool neither absorbs much radiation but presents a large surface to volume ratio, and hence keeps cooler. Lakes here
keep coolish by evaporation. Latent heat of evaporation is very high for water.
@not_important. – The absorption spectrum of liquid water is quite different than for vapor. See
http://www.lsbu.ac.uk/water/vibrat.html
for a very good discussion of the properties of that unique substance, Water. Good graphs there.
The above ref. is also recommended to all global warming nuts. After all, H2O is the chief greenhouse gas – maybe we should ban it, Mr. Gore.
… and Rosco, I suggest you take steps to limit the emission of urea into your pool. With hypochlorite, it forms chloramines, nasty lacrimators, and
possibly the dreaded NCl3, though I’ve never yet heard of an exploding pool!
Regards,
Der Alte
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
| Quote: | | The above ref. is also recommended to all global warming nuts. After all, H2O is the chief greenhouse gas – maybe we should ban it, Mr. Gore.
|
Yes liquid spectra are much broadened by molecular interaction, in h2o esp due to hydrogen bonding - can see it in ftir spectra. But that graph is an
excellent example of greenhouse gasses. Notice how much more H2O absorbs. from FTIR spectra I posted you can see how narrow the CO2 absorption peaks
are.
And it forms less than 0.01% of the atmosphere, water is much higher. i have not run climate change models but suspect this might be a storm in a tea
cup for the sake of the fame and fortune of a few - we need more causes
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Yes, liquid and solid phase absorption bands are broader, shifted, and more intense. But if you'll notice in liquid phase charts the strong absorption
is at wavelengths longer than 1 micrometer, and from the chart I posted the majority of energy content of sunlight is at shorter wavelengths.
The steep slope of the IR absorption also results in most of the IR being stopped in a relatively thin surface layer, with the peak of solar flux
penetrating for meters. As a result the light in the peak flux range bounces off light colour walls and bottom and back out of the pool.
A note on CO2, H2O, and climate. Water vapour and carbon dioxide act differently, because water condenses at higher temperatures it is mostly
concentrated in the lower atmosphere while CO2 is more uniformly mixed. As a result CO2 dominates the transmission in the upper atmosphere, which
means light reflected from clouds and radiated from those clouds as water condenses there. Also CP2 'plugs' two holes in the H2O bands, right in the
maximum flux range of black body radiation at temperatures related to the Earth's surface and clouds.
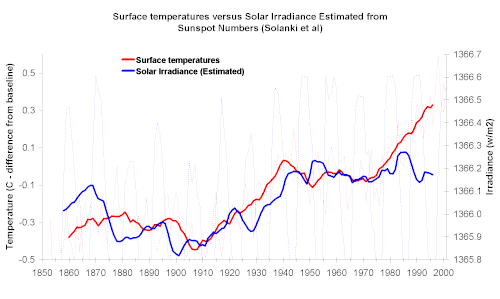
And so far it is the CO2-based climate warming models, which do include some effect of water, that best explain data such as that in the graph below,
although the 2005-2010 data will be an important test of those models.
[Edited on 2-8-2008 by not_important]
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Thanks for the graph not_important. I will however have to disagree on its interpretation.
First one must understand that to superimpose two different quantities on a graph both offset and scale are adjusted willy-nilly, and the only
consequence are the trends. I fail to see any short term correlation in the two curves at all. As for long term trends irradiance correlates with
the 0.25C rise 1900-1950, but fails to correlate with the 0.25C rise the last 30 years where most of the CO2 has been output and of which the fuss is
all about. Presumably the CO2 models try to make up the difference - Ill just point out that the deviation now from radiation mean is not that much
more than a standard deviation, and differences in solar radiation levels seem to be more substantial effects.
Also note the variation in irradiance is about 0.02% in total - and we are supposed to know to that accuracy how CO2 alters the atmosphere energy
balance and how it contributes to average surface temperatures! Thats the whole problem people have with these climate change models.
The ( earth )black body radiation is very broad and CO2 captures only a small part of it. In fact the atmosphere is pretty opaque in IR (people doing
FTIR will know what I mean its hard to get anything that will transmit there), and so the climate deductions are not just simple absorption length
caluclations but depend intricately on mass transfer and re-reflection between layers to caluclate the final outward radiation flux from the earth.
And this they purport to do to an accuracy of 0.02%!
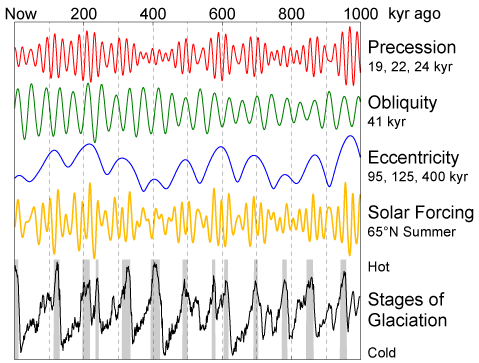
I will also add that its a myth that the earth has had its CO2 and temperatures shifted well out of ordinary by man. Earth temperatures oscillate by
as much as 10C!! on average with a cycle of 400000 years if I remember correctly. We are currently getting out of an ice age - 10000 years ago north
europe and north america were covered by glaciers - the traces of which are still obvious. Most of the time the earth existed (in the biological era)
the poles have been free of ice - lizards were able to live at 5 degree latitudes from the poles. In the time of the dinosaurs CO2 content was up to
30 times present and the temperature on average much warmer. And these fluctuations happen by natural causes. By comparison if we burned all fosil
fuel we can lay our hand on the world temperature (also predicted by these models will rise 2C). So if we think we can fight large natural
alterations with our small contribution in any long term way we should have another think. Needless to say at the time of the dinosaurs the red and
blue lines would be in complete discord.
Scientists are losing jobs world over, physics for instance is rapidly running out of 'newities'. Its natural that those at risk will think of some
calamity to safeguard their jobs. This is of course simplified, but it is highly naive to thibk that scientists are objective people - theyre like
the rest of us.
[Edited on 2-8-2008 by len1]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
For much of those warm periods at least one pole had free oceanic circulation, and the apparent cO2 levels were quite high http://www.globalwarmingart.com/wiki/Image hanerozoic_Carbon_Dioxide_png hanerozoic_Carbon_Dioxide_png
(not the best source but also not behind pay walls)
we're in a cool and low CO2 concentration era, both have been falling (on the average) for the last 10 million years.
Those climatic oscillations are related to the Milankovitch cycles, with a number of frequencies interacting.
We've been out of an ice age for some time. The Holocene climatic optimum started some 9 to 10 thousand years ago, and continued until some 5
thousand years ago. After that it cooled slightly and slowly until about 2 thousand years ago, leveled off until around 800 CE and the (not global)
Medieval Warm Period, lasting until around 1300 CE. Between the 16th and late 19th centuries was the Little Ice Age, also not global although more
widespread than the MWP, with three cold intervals; however this was not nearly as severe as conditions before the HCO and appears to be one cycle in
an oscillation that started with the HCO.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
DerAlte, my curiosity is more scientific than practical. I send you a PM with details.
The posts here are very informative and it is clear to me that I overestimated water's IR absorption. The insulation and evaporation factors also
contribute but, since some regular pools stay warmer than the ambient using only solar panels, I think it's mostly a matter of efficiency in absorbing
solar energy.
One more thing: not_important points out that about 50% of the sun radiance comes in the UV and visible band. Well, I may sound ignorant here, but I
can't thing of a black surface being heated by UV or visible light. I know it should, since energy has to go somewhere, but a fluorescent light
doesn't strike me as heat source, no matter how bright. The same number of watts on an incandescent light on the other hand...
Am I 100% wrong here or is there some physics principle that makes 100Wh of IR be more efficient than 100Wh of purple light in heating a (real world)
black surface?
[Edited on 2-8-2008 by Tacho]
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
I think Rosco is illistrating caffeins' diuretic properties and pool patrons will bore easily of the constant restroom visits. So to limit
interuptions in pool fun...the patrons can contribute to the esquisant character that salt/urea/ and phosphates add to pool tea flavor 
Fellow molecular manipulator
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | I can't thing of a black surface being heated by UV or visible light. I know it should, since energy has to go somewhere, but a fluorescent light
doesn't strike me as heat source, no matter how bright. The same number of watts on an incandescent light on the other hand...
Am I 100% wrong here or is there some physics principle that makes 100Wh of IR be more efficient than 100Wh of purple light in heating a (real world)
black surface? |
A T8 fluorescent light is typically 32 watts, spread out over 4 feet on length. An incandescent lamp is going to have all its power dissipation
concentrated roughly 1/20 the distance, and a 40 watt incandescent is not a large wattage for tungsten bulbs so you're looking at 25 times or more the
power density. To get a fair comparison you'd to make a line of 1 watt incandescent bulbs spaced so as to be as long as the T8 bulb, roughly every 3
cm, or efficiently focus the output of that T8 onto an elliptical spot 5 cm in its longer axis.
|
|
|
Twospoons
International Hazard
    
Posts: 1281
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Yes, it really is all about energy density. You wouldn't expect to feel "heat" off an LED, but I have. It was an LED based glue curing gun (for UV
set glue) I built using 3 x 5W deep blue LEDs (455nm). The total light flux was around 1.5W: it was really nasty to look at, and you could feel the
"heat" on your hand easily - even though there was no IR output from the LEDs.
I should add that just because something looks black in the visible spectrum does not mean that it in black in other parts of the spectrum! This is
used to great effect in selective absorbers in solar heating. I even have black loudspeaker grill cloth that looks white to IR.
[Edited on 4-8-2008 by Twospoons]
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
-jeffB
Hazard to Others
  
Posts: 185
Registered: 6-12-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Tacho
One more thing: not_important points out that about 50% of the sun radiance comes in the UV and visible band. Well, I may sound ignorant here, but I
can't thing of a black surface being heated by UV or visible light. |
YouTube is full of people popping balloons and such with lasers. You can't get much more visible (with no IR component) than that!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
If a bit of calculation in translation is done, every sort of energy quanta can be expressed as heat units, like calories, and energy absorbed and not
used to perform some other work as a result, is going to manifest itself as heating, a positive change in enthalpy, in obedience to the fundamental
law of thermodynamics.....Correct ????
So the wavelength (resonant frequency) of incident energy absorbed is totally
irrelevant ...so long as it is absorbed by the target material, then that target material is going to heat up accordingly, and directly proportionally
to the "heat quanta" associated with the intensity of the impacting radiation.
Behold the universe, it is all heat in some form or another.
Therefore, it follows that wherever you go, there you are,
and there some heat is always with you . .
Max Planck, front and center to the podium please
http://en.wikipedia.org/wiki/Planck%27s_constant
Okay philosophers, have a ball,.... would it be a photon?    Once there was a particle theory, Once there was a particle theory,
but it just made waves every time it was examined, or did it?
[Edited on 4-8-2008 by Rosco Bodine]
|
|
|