Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Taming Phosgene
In a recent thread on Toxicity of Phosgene a number of phosgene substitutes were discussed, such as trichloromethyl chloroformate (diphosgene) and
hexachlorodimethyl carbonate (triphosgene).
The problem with diphosgene is that, while less volatile and way less insidious than phosgene, it is far from harmless.
And the problem with triphosgene is that, although solid and almost nonvolatile, it still releases phosgene in situ and thus a phosgene hazard exists
in workup.
In terms of reactivity phosgene > diphosgene >> triphosgene and the latter does not always react as expected.
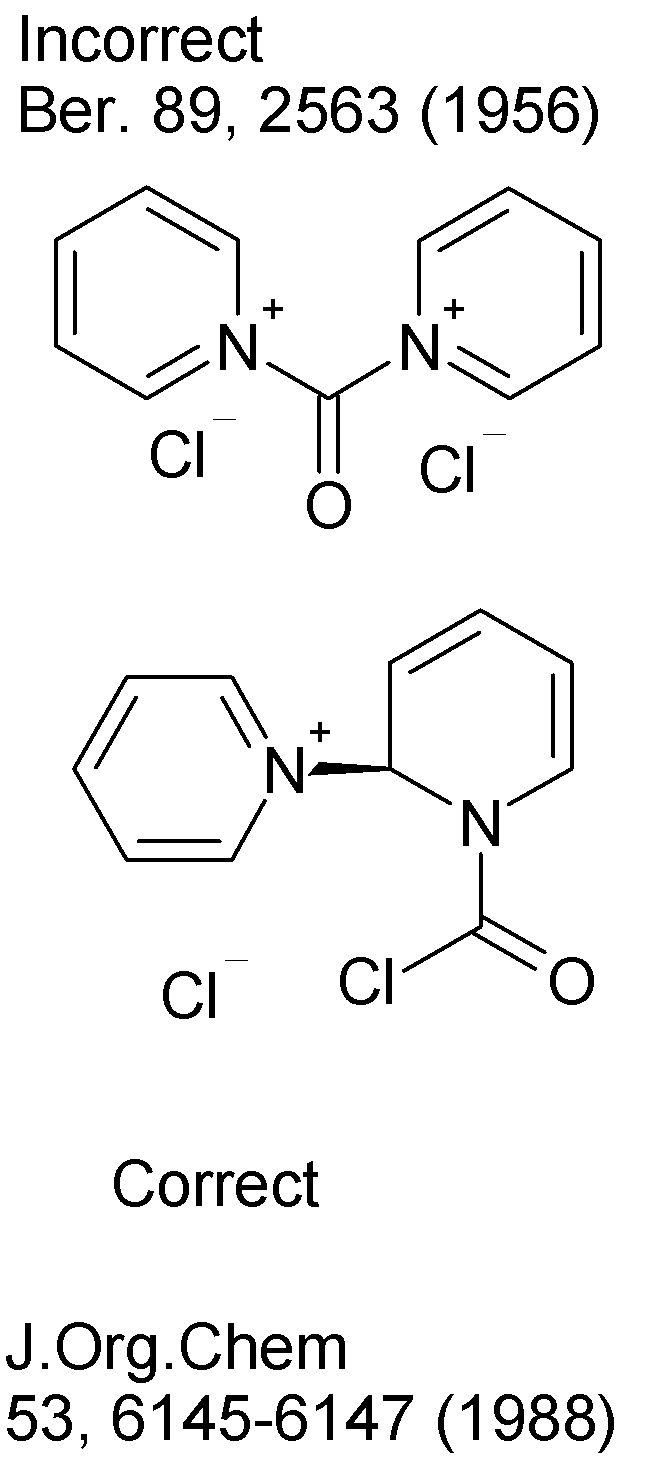
I ran across an alternative phosgene substitute, or rather a pair of them: the little known solid salts of phosgene and pyridine.
As Ozone pointed out, phosgene is a lot less scary in solution than it is as a neat, condensed liquid or vapor over liquid in a lecture bottle or
quarter tank.
Toluene is a common solvent for phosgene and will solvate 20% of its weight.
The pyridinium (1:1 adduct) salt of phosgene and the dipyridinium (2:1 adduct) salt are prepared by reacting the two at 0-4 C in a nonpolar aprotic
solvent. Pentane, ether, benzene and toluene are all suitable. The resulting flocculent precipitates are only sparingly soluble in these, and are
stable at < -10 C so are stored in SureSeal or equivalent bottles in the lab freezer. They can be transferred by syringe techniques or transfer
line.
I will describe the 2:1 adduct first as I am still gathering details on the 1:1 salt.
This is a light yellow to canary yellow microcrystalline powder. As [prepared it contains occluded pyridine which is removed by 24 hrs vacuum at 1 to
30 torr. It is extremely hygroscopic and slightly photosensitive. Therefore I would recommend leaving it in slurry form in toluene.
The salt at temperatures above - 10 C readily decomposes to pyridine (2 Mols) and phosgene, reversibly. The advantage of keeping in toluene slurry is
that even if this is allowed to occur, the phosgene will remain in solution in the toluene. Rechilling the solution regenerates the slurried salt.
Therefore, the slurry of this dipyridinium salt is safer than a solution of phosgene per se, as long as it is kept at -10 C or below.
The salt can be directly substituted for phosgene in reactions. For example one mol of this salt and 2 mols aniline give a quantitative yield of
diphenylurea.
The original investigator assigned a simple double quaternary salt structure on the basis of stoichiometry, but the matter was re-examined by 13CNMR
and the structure was found to be quite different.
[Edited on 15-8-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Combined with the relative ease of preparation of phosgene from i.e. CCl4/CHCl3 and oleum, this should make it rather workable: Non-pressurised
storage of OCCl2 in a normal household freezer, and immediate in situ reaction with target molecules - brilliant. One'd suggest to try dinitrophenyl
hydrazine for testing its reactivity, if aniline is not at hand 
| Quote: | | Toluene is a common solvent for phosgene and will solvate 20% of its weight. |
More seriously - clearly the basis for solution of phosgene into pyridine is different than that with aliphatic hydrocarbons, or benzene derivatives.
I didn't quite catch the advantage of using pyridine vs toluene etc? Vapour pressure perhaps?
[Edited on 15-8-2008 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Ethyl acetate is a better solvent for phosgene than toluene, there is a hilarious paper avalible:
Solvents for Phosgene.
Charles Baskerville, P. W. Cohen
Ind. Eng. Chem.; 1921; 13(4); 333-334.
I can't access it at the moment but the author is trying to find a good solvent for phosgene because it is too dangerous to transport it as a
compressed gas and there is a surplus of it after WWI.
The author comes to the conclusion that ethyl acetate and benzene are the best solvents, both dissolving greater than 50% by weight phosgene with no
reaction. The author further elaborates that benzene is cheaper so it should be the solvent of choice. Finally, the author attempts to set forth a
use for the 50% phosgene in benzene solution, to apply it to ones lawn to kill moles and gophers.
I'd never herd of these complexes of phosgene but they do offer some alternatives to the neat compound and the di- and tri- phosgene derivatives.
Reminiscent of the SO<sub>3</sub> complexes with pyridine and such. It's a shame they have to be kept at sub-zero temperatures but it's a
small tradeoff since a commercial freezer will easily maintain those temperatures.
Another thought, although still fairly toxic, thiophosgene can accomplish analogous transformations (and at a much cheaper price) and in some cases
such as isothiocyanates, the products can be air oxidized to the desired isocyanates, essentially giving the same overall reaction as if phosgene had
been used (thiosphosgene itself will oxidize in air to phosgene as well).
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
| Quote: | Originally posted by BromicAcid
Finally, the author attempts to set forth a use for the 50% phosgene in benzene solution, to apply it to ones lawn to kill moles and gophers.
|
Doubtless it would finish off worms, cockchafers, crane fly larvae, wireworms etc as well and the effects on the spouse, offspring and pets etc could
be fatal.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
When I first encountered the papers on these salts I had hopes of applying this to a solid support such as poly(4-vinylpyridine) (PVP resin beads) but
the hygroscopicity, low temp requirement and inconvenience of having to use phosgene solution to regenerate the resin after use, all mitigate against
the utility of this approach as well as the additional cost.
I would not contemplate using a household freezer, IMO a lab freezer rates explosion proof is the only way to go, despite the expense. If you are
going to store volatile flammable solvents in an ordinary refrigerator-freezer you are asking for trouble.
The solution would contain three components: the solvent, phosgene and pyridine in a 5:1:2 ratio (the phosgene-pyridine by molarity, the
toluene-phosgene by weight.) And the container must be either a SureSeal or equivalent one designed for gas tight performance and access to contents
by syringe. In this fashion, the contents are protected from moisture, and the lab is protected from phosgene even in event of a power failure, and
rise of temperature inside freezer to ambient.
Alternatively, a lecture bottle or sample bomb or Kilo-Lab style storage would be an option, again with access via syringe through an opened valve. As
there will be no pressure, there would be little opportunity for escape of phosgene during tranfer, as it will be bound up first in the salt and if
not, then is the solution.
The overall strategic advantage is that generation of phosgene would only need to be done once or at most occasionally, with concommitent need to soak
the glassware involved in order to destroy phosgene clinging to the inside surfaces. Needless to say, the generation, cleanup, and all dispensing of
the resulting solution/slurry needs to be done exclusively in the hood.
If we are talking toluene for solvent then a one liter solution of the salt/slurry would contain 2 mols. A 2.5 L bottle, 5 mols. If you are
performing experiments on a 10 to 100 mmol scale, this will last a good long time before need to generate more phosgene.
The most competitive alternative strategy is triphosgene, which requires a rather costly fairly large scale (say 10 L) photochemical reactor and quite
a bit of CCl4 to prepare, and which does not always work well as a phosgene substitute due to lower reactivity. As triphosgene still generates
phosgene in situ there is still same phosgene hazard during workup, as Ozone and another member pointed out elsewhere. The big advantage of
triphosgene is that there is no need to prepare or handle phosgene at all at least till workup.
(The photochemical reactor and UV irradiation required to make triphosgene pose their own set of safety issues to be dealt with as well.)
Thiophosgene is indeed an interesting reagent but not always entirely analogous to phosgene in its reactions much less equivalent. Phosgene for
example gives isocyanates from primary amines, thiophosgene gives isothiocyanates or thiocyanates. Thiophosgene is rather a pain to prepare, the
options being pretty much limited to chlorination of CS2 followed by reaction of CCl4S (CCl3SCl) with alumina, or chlorination of MeSCN. The
substrates are not cheap. The former reaction produces a great deal of hot angry H2S, while the latter is relatively low yield since CSCl2 is but one
of the products. Both produce a lot of sulfur chlorides as well. Which is fine if you have a use for them, otherwise they are a stinking, toxic,
corrosive pain in the ass.
As to benzene or ethyl acetate as phosgene solvents, the former is a lot more hazardous than toluene and the latter, I am not sure is entirely inert
to phosgene chemically. Let me look into this. No gophers around here, though plenty of go-fers. I will look at the lit.
--------------------
A quick look at SArtori gives the following solubility data which is taken from same Baskerville & Cohen paper as mentioned above:
Benzene 1 Kg dissolves 1 Kg
Toluene 1500 g dissolves 1 Kg
Petrol 1200 g dissolves 1 Kg ('d take this to be same as petroleum ether)
Chloroform and glacial acetic acid were almost as good. CCl4 was half as effective as tolene (go figure.) Sartori did not mention ethyl acetate.
According to Sartori 30% phosgene in toluene was marketed commercially.
Ethyl acetate (and esters in general) are often used as solvents for phosgenations. However the devil is always in the details.
Under certain conditions, alkyl esters of carboxylic acids are split to an acyl cgloride and an alkyl chloride by phosgene and HCl, AlCl3 or
(significantly in the present case) phosgene and pyridine. See p.82 of the attached review of phosgene chemistry from Chemical Reviews, and references
cited therein.
Therefore I think ethyl acetate is out as a potential solvent for the phosgene-pyridine system
Sorry about that!
The particular paper cited is Ref 163, which happens to be one I have already in full text. It is US Patent 2,778,852. Ref 162 is the corresponding UK
patent to same inventor. If anyone is interested I will post it in a subsequent post.
[Edited on 15-8-2008 by Sauron]
Attachment: cr60281a005.pdf (369kB)
This file has been downloaded 7047 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I am not sure when it stopped being a commercial product but 60% phosgene in toluene was still hanging around out lab in the early eighties. The
bottles were quite old and stored in a deep freeze that nobody opened very often.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Here's the 1956 paper from Ber. describing the solid, yelloe microcrystalline 2:1 salt of pyridine and phosgene. Remember, ignore his ideas about
structure as they were proven wrong 32 years later. Well, I guess he didn't have an NMR handy in 1956.
-------------------
I may have overstated the case against EtOAc as solvent for the pyridine-phosgene slurry and system. But not by much.
Per patent, reaction conditions were minimum temp 80 C with a few mol% pyridine as catalyst, to get phosgene to cleave an ester to acyl chloride and
alkyl chloride.
Preferably more like 120-140 C which for EtOAc would mean autogenous pressure.
It is anybody's guess as to whether or not this reaction might take place in the cold, slowly, with a much higher amount of pyridine present.
However, it seems to me that given other much more inert solvents such as benzene and toluene there is simply no great need to find out. Prudent just
to skip esters as solvents for this system.
[Edited on 16-8-2008 by Sauron]
Attachment: dipyphos.pdf (272kB)
This file has been downloaded 704 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
If anyone's interested...
Solvents for Phosgene.
Charles Baskerville, P. W. Cohen
Ind. Eng. Chem.; 1921; 13(4); 333-334.
+++++++++++++++++++++
A little question: doesn't the dipyridinium phosgene salt still have to be used as soon as prepared? I don't see it lasting long even if kept at the
recommended temperature.
(Then again, this is not something one should have in sizable quantities!)
sparky (~_~)
Attachment: phossolv.pdf (265kB)
This file has been downloaded 715 times
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The phosgene salts (1:2) of trialkylamines like TEA must be used ASAP because they decompose to sym-dialkylurea with extrusion of alkyl halide.
Pyridine can't do that. According to the literature, this 2:1 pyridine-phosgene salt is stable as long as it is dry and as long as it is kept at
<-10 C and out of strong light. This is regardless of whether it is stored in slurry, or as a solid. If as a solid, it must be pumped down for a
day to remove occluded pyridine.
As a solid, it would be a hazard if temperatures were allowed to rise to ambient, such as in event of a power failure. It would revert to pyridine (2
mols) and phosgene. So the only safe way to store the solid would be in a pressure vessel.
As a slurry in a nonpolar aprotic solvent with a high capacity to solvate phosgene, this scenario can't happen. If a mol of the salt were slurried in
500 ml of benzene or toluene for example, in event of a power or refrigeration failure, one mol phosgene (100 g) and two mols pyridine would form
(c.170 g) while the capacity of benzene to solvate phosgene if 500 g in 500 g and that of toluene is 200 g in 500 g. Thus, no pressure would develop.
A number of options exist for containment. These range from SureSeal (Aldrich) type gas tight glass bottles, to mild steel Kilo-Lab type steel
cylinders, which are DOT rated and threaded for valves, to stainless steel sample "bombs" also DOT rated and set up for valves and gauges. Using a
simple valve, aliquots can be drawn by SS or PTFE syringe needle. I use a 100 ml Hamilton gas tight syringe for this sort of work.
Those options are listed in ascending order of cost but also ascending order of physical integrity. You get what you pay for.
There are unmentioned intermediate options such as mild steel and SS lecture bottles. These have limited capacity, but are relatively cheap and are
stronger than Kilo-Lab steel bottles. I'd always opt for SS and a monel or SS valve because mild steel is not going to be happy with COCl2. But a lot
depends on how long one plans to store the slurry. The only reason to prepare the slurry is to use it, in a moderate amount of time. If you want long
term storage of a phosgenating reagent, opt for bis(trichloromethyl) carbonate (triphosgene) instead. But why would one want long term storage? I
woild think making a few mols, and then using those in experiments of up to 100 mmol, in the space of weeks, would be about the right tradeoff. It's
not something you need off the shelf when you are not doing work that requires this reagent.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
And another paper on this matter, this one primarily on the TEA-phosgene 2:1 saltsystem but also discussing pyridine-phosgene salt.
--------------------------
At the risk of overstating the obvious, I think the best way to store phosgene is not to store it, or rather to store it as triphosgene, from which
three mols phosgene are available from one mol hexachlorodimethyl carbonate.
Guideline 1: if there is any way to get the product you want by any other means - do it.
Guideline 2: if you must use phosgene then try triphosgene and make phosgene in situ. This usually works, though not always. Triphosgene reacts
sluggishly compared to phosgene or diphosgene and sometimes, reactions just fail.
Guideline 3: If triphosgene fails, then use triphosgene to prepare phosgene solution and react that with pyridine, then use the slurries salt to
react.
Guideline 4: Only as a last resort use phosgene in solution directly.
Guideline 5: If procedure calls for neat liquid phosgene, ask yourself if you REALLY need to do that procedure?
If you follow these guidelines you will maximize your chances of getting the job done at the least possible risk.
The literature says the 2:1 salt of pyridine and phosgene is soluble in DCM and MeCN. Unfortunately Baskerville and Cohen were mum about the
solubility of phosgene in these solvents. The problem is that my premise that the slurried salt can be drawn by syringe is that the slurry is not
going to remain homogenous, the salt will settle out, and therefore aliquots can't be expected to contain a uniform amount of the salt. Using MeCN
or DCM would result in a solution of the salt, but then if the temperature is allowed to rise, phosgene will form and the pyridine and solvent mix may
not hold it - we can't know without determining the capacity of the solvent experimentally. Not so convenient.
Therefore, I'd prepare the salt as needed rather than storing it.
True, the DCM or MeCN solution of the salt can be stored safely in a pressure vessel (LB, etc.) fitted with a phosgene-compatible regulator but doind
so would have scant advantage over storing compressed (gas over liquid) phosgene, particularly if cold storage could not be guaranteed.
So triphosgene is best "storage" or unstorage. Nascent phosgene if you will.
So we are back to the UV catalyzed chlorination of dimethyl carbonate in CCl4 on a prep scale.
[Edited on 17-8-2008 by Sauron]
Attachment: trimetphos.pdf (282kB)
This file has been downloaded 734 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
|