| Pages:
1
2 |
fuming_nitric_acid
Harmless

Posts: 19
Registered: 23-7-2008
Member Is Offline
Mood: No Mood
|
|
Esterification of Malonic Acid to DiEthyl Malonate
How can this esterification be done? Experimental procedure please.
Thanks!
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
...............you must make the effort to find something yourself.....I just logged into google with your question and the answer is right
there....so no spoon feeding ......solo
........since I looked it up here is one bit of info,
http://www.chemyq.com/En/xz/xz1/7855pnrie.htm
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
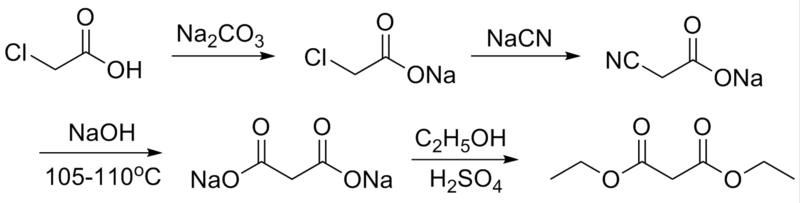
This is the general synthesis scheme.
Chloroacetic acid (MCA) & its sodium salt (SMA) may still be used as herbicides & may be available OTC. Sodium carbonate & sodium
hydroxide are OTC. Sodium cyanide, though, might get you a visit from the FBI. An alternate starting material would be either cyanoacetic acid or
sodium cyanoacetate.
The process is described in this patent: http://www.pat2pdf.org/patents/pat2337858.pdf
An alternative to using ethanol & sulfuric acid in the last step is to react the disodium malonate with ethyl chloride in a solvent under
pressure. Ethyl chloride is sold as a skin cooling agent but it is Rx.
[Edited on 23-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
This reaction is detailed exhaustively, with references, in Org.Syn.
In a moment I will post the pdf.
As Ritter says OS is upgefucked. The prep is however in CV 2 p.376.
It is also, in abbreviated form in Vogel's Preparative Organic Chemistry 3rd Edition which is in forum library. There'a a whole section on malonic
ester and acetoacetic acid syntheses.
Ritter gave the sequence properly but there's a lot of fine print you will want to know.
[Edited on 24-8-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
This reaction is detailed exhaustively, with references, in Org.Syn.
In a moment I will post the pdf. |
The Org Syn site is having problems. I tried searching there earlier but some of the search functions are disabled.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Ludwig to the Rescue
The prep from chloroacetic acid is described on pp 161-162 of Gattermann, attached below (from forum library.)
What the thread author asked was actually how to esterify malonic acid, not how to prepare it. The esterification is the last step before workup, and
as any tyro ought to know, is effected with an excess of anhydrous (absolute) ethanol and catalytic conc H2SO4.
Org.Syn. is working again.
[Edited on 24-8-2008 by Sauron]
Attachment: Pages from the_practical_methods_of_organic_chemistry.pdf (197kB)
This file has been downloaded 1362 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
fuming_nitric_acid
Harmless

Posts: 19
Registered: 23-7-2008
Member Is Offline
Mood: No Mood
|
|
Yes you're right Sauron. I need the experimental procedure of how to esterify malonic acid not how to prepare him! Because I wonder how the produced
water is driven off fom the reaction.
Thanks to you all...
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
You wonder how water is "driven off" in presence of concentrated H2SO4?
Think about it.
Then read Vogel's introduction to this chapters on aliphatic esters, dicarboxylic acids, and finally, malonic and acetoacetic acids.
You mix malonic acid with excess (3-4 mols per mol acid) absolute ethanol then you slowly add, with stirring, concentrated H2SO4.
Acids catalyze esterification. A little p-TsOH would also work, as would bubbling dry HCl gas through.
Now would you like to hazard a guess how you would recover malonic acid from its ester? Breaking up an ester into acid and alcohol is called
saponification. What sort of compound drives saponification do you reckon?
a. acid
b. base
c. Unobtainium nonachloride UnCl9
d. none of the above
Sic gorgeamus a los subjectatus nunc.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
e. All of the above. 
Incidentially, acid or base catalyzes cleaving an ester, base just works better. And why is another excellent question to be answered. 
Tim
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Here's Vogel p 483-485. Maybe clearer than Gattermann's verbage. Also he recites two different mechanisms, only one of which actually involves malonic
acid per se as an intermediate.
Not being much of a mechanic I do not recall which is now the preferred mechanism by contemporary lights.
AFAIK esterification of malonic acid is just a typical procedure akin to esterifying oxalic acid or succinic acid. That being said, I know that
malonic acid has some idiosyncratic properties. For example, while TCT readily forms oxalyl chloride from oxalic acid, HOOC-COOH, and siccinyl
chloride from succinic acid HOOC-(CH2)2-COOH, the reaction fails for malonic acid HOOC-CH2-COOH. Why? There does not seem to be any malonyl chloride.
(If there were, it would be a neat way to make diethyl malonate.)
Malonyl dichloride, the acyl chloride of malonic acid does exist. Aldrich sells it in 100 g ampules. Acros does not sell it. This is interesting.
There's a ref to Beilstein 2, IV. Was this only prepared in the second half of the 20th century? I will have to look this up. I wonder how they made
it. TCT failed, I would guess SOCl2 would also. PCl5 maybe? Why in ampules? Is it so air/moisture sensitive?
[Edited on 25-8-2008 by Sauron]
Attachment: Pages from vogel_practical_ochem_3.pdf (309kB)
This file has been downloaded 1971 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Introduction
I just finished a synthesis of diethyl malonate (DEM) using malonic acid in a Fisher esterification . I reported the synthesis of the acid in a
previous thread, "Oxidation of Diols."
DEM seems to be a very useful intermediate. For example, it can be used to make a carboxylic acid with 2 additional carbons starting with an alkyl
halide. My old organic lab manual (Brewster et al, 1962) uses it for making caproic acid.
Theory
CH2-(COOH)2 + 2C2H5OH ---- CH2-(COOC2H5)2 + 2H2O
The yield limit on most Fisher esterifications is about 67% due to equilibrium considerations. To increase this yield one of the reactants can be
used in large excess and/or one or more of the products can be removed during the rxn. Both of these techniques were used in the following synthesis.
Reference
American Journal of Science, 1908, 4th series, Vol. XXVI, no. 153, Supplement: Art. XXV "On the Esterification of Malonic Acid," by IK Phelps
and EW Tillotson, Jr.
Specifically, the technique of Table II, C, 2, was used. In this case the authors reported a yield of 96.11%.
Procedure
a. reaction
15.4g malonic acid, 65 mL anhydrous ethanol, and 0.6mL conc H2SO4 were combined in a 250 mL RBF. This was placed in an oil bath in a simple
distillation set-up.
Along side that was placed a 500 mL RBF with 130 mL of anhydrous ethanol. This was used as a vaporizor, sparging ethanol vapor into the
esterification pot.
The oil bath was set at 105C. As the alcohol/water azeotrope was distilled off it was replaced with alcohol from the vaporizer. This removes water,
a product of the reversible reaction. This was continued until all the alcohol (65mL + 130 mL - that reacted) had distilled over.
b. workup
The 35 mL in the pot was placed in a sep funnel, chased out with a little DCM (I didn't have any ether). About 40 mL of sat aqueous Na2CO3 was added
until the mix was slightly basic. ~35 mL of DCM was then added to extract the DEM. The water layer was discarded. This was then washed with ~40 mL
of sat aqueous NaCl, discarding the aqueous layer.
c. vacuum distillation
The DCM/DEM extract was placed in a 100mL RBF for vacuum distillation. The oil bath was set at 60C then 70C until all the DCM and a little water had
distilled over. Vacuum was 16" Hg. Then the bath temp was raised to 140C and the DEM came over at 91-104C. Vacuum was 27.5" Hg.
Results
12 mL of DEM was produced for a yield of 53%.
Conclusions
Although the intended product was produced in reasonable purity, the yield is disappointing. I'm skeptical that the alcohol vaporization accomplished
anything. Increasing the batch size would no doubt improve yield by reducing mechanical losses. Ether might have been a better extraction solvent
than DCM.

[Edited on 15-10-2009 by Magpie]
[Edited on 15-10-2009 by Magpie]

[Edited on 15-10-2009 by Magpie]
[Edited on 15-10-2009 by Magpie]
[Edited on 15-10-2009 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
grind
Hazard to Others
  
Posts: 120
Registered: 13-1-2007
Member Is Offline
Mood: No Mood
|
|
Malonic acid + 5 moles of 95% ethanol + CHCl3 + a trace of TsOH at the Dean-Stark-Trap. Workup = only distillation, no need for washing, extracting
and so on. Yield should exceed 90%. Works excellently with oxalic acid (I produced diethyl oxalate in this way) with the only exception that no
catalyst is needed.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Good idea grind. I've never used a Dean-Stark system before. This looks like a justification for getting some equipment.
I don't have much chloroform. But I see in my handbook that benzene forms a low boiling ternary azeotrope with water+alcohol also. That I have.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
DCM also forms an azeotrope with water, although you need a dean stark for heavy entrainers. b.p. is about 38C IIRC.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
I think you can substitute the benzene for toluene.
Better for health, and nicer smelling.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Magpie: I think you may find that the amount of water produced in a Fischer Esterification reaction is rather negligible and need not be removed to
drive the reaction entropically. I wouldn't bother using a Dean Stark trap, which, by the way, won't work terribly well unless you use toluene or
benzene, which aren't an option as their BP's are above that of ethanol. Easier to use excess ethanol, ~2.2eq of H2SO4 and reflux for maybe an hour
or two. The 2 mole equivalents of water that are generated are not really sufficient to drive the reverse reaction. Think about it. If you want to
cleave an ester, you can use 1mole eq. of base, but usually use use an excess of water. As was mentioner earlier, acid hydrolysis of esters is also
slow. I think procedurally this would be easier. On another note, you might improve your yield by altering the workup. I would advise using
saturated NaHCO3 (saturated Na2CO3 is quite a strong base), and add the DCM or EtOAc to the crude reaction first, then wash with base, water, dry with
MgSO4 and evaporate. This way you avoid hydrolyzing any product. I should also think that the product obtained thereafter might be sufficiently pure,
making a vacuum distillation unnecessary.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks for the reminder Jor. I always prefer to use cheap and readily available toluene in lieu of my precious benzene. 
Arrhenius:
The bp of the benzene/ethanol/water azeotrope is 64.9C. So why wouldn't this work in the Dean Stark?
I see that you recommend 2.2 equivalents of H2SO4. Mimicking the old reference I cited above I used 0.14 eq H2SO4. I thought this seemed quite low
based on current practice for esterifications.
Thanks for the suggestions on a better workup.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
If you form a Schiff base or something like this, and you use benzene or toluene, you'll collect a small amount of water in the DS trap, and you don't
really need to empty out your trap. You just run the reflux for a few hours. If you're azeotropically distilling and you're forced to remove the
azeotrope and cannot return it to the reaction (e.g. benzene/ethanol/water), then there's no sense using a DS trap. It may be possible to put
molecular sieves or some drying agent in the DS trap, but I've never actually done this. If you're going to empty the trap frequently you might as
well use a simple distillation setup as you have done already. Make sense? I only suggested 2.2eq of acid catalyst because this is typical for Fischer
Esterification, but I have not done so to make diethylmalonate.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Does water separate from the benzene/ethanol phase? If not I see where the Dean Stark would be of no advantage over simple distillation.
The authors of my reference in my 1st post did experiment with using 0.34 equivalents of H2SO4, but it showed no improvement over 0.14 equivalents.
Perhaps this is something specific to malonic acid. It is very vulnerable to decomposition, which in the end is one of the keys to its utility.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Does water separate from the benzene/ethanol phase? If not I see where the Dean Stark would be of no advantage over simple distillation.
|
See attached.
| Quote: | | The authors of my reference in my 1st post did experiment with using 0.34 equivalents of H2SO4, but it showed no improvement over 0.14 equivalents.
Perhaps this is something specific to malonic acid. It is very vulnerable to decomposition, which in the end is one of the keys to its utility.
|
I'm used to seeing fractional equivalents, too. The exception is when the H2SO4 is being used to tie up the water generated.
You can run an azeotropic system in an extractor, with a drying agent in the extraction section. This allows using less of the added azeotroping
agent, and can be handy when making medium sized batches of esters as you don't get the large volumes of distillate. Molecular sieves can be used; in
some cases MgSO4 or even Na2SO4 will work, especially if the extractor can be run so that the condensed liquids are cool to cold which increases the
stability of the hydrated salt.

|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Which mol. sieves do you use with alcohol? Interesting table, I'll try to remember that.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you for the information and data, not_important. Although not real efficient, it looks like using a Dean Stark would be worthwhile.
[Edited on 20-10-2009 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
3A sieves, 4A starts to pick up methanol and to a lesser degree ethanol.
Yes, the bz-EtOH-H20 azeotrope isn't a great one. Besides the ratios, the densities are close enough that droplets of the aqueous phase can get
carried over in the organic phase. But you just let it keep recycling and all the water eventually gets removed, once you get the input heat flux
right the setup requires little attention.
|
|
|
tiger1
Harmless

Posts: 8
Registered: 13-4-2008
Location: Left Coast
Member Is Offline
Mood: Mojo Rising
|
|
Came across this thread via the SE with Malonyl Chloride. Per Sauron's idea I'm wondering if anyone has ever tried BzCl (Benzoyl Cl) to prepare the
Malonyl Chloride. BP of Malonyl Chloride is low at 53-55 so should distill out fairly easily and a small amount of base in pot would scavenge HCl. I
could almost envision after a small forerun of Malonyl Chloride, replacing the receiver with a flask of anhydrous ROH and if stirred, cooled, and N2
flushed, could simultaneously carry out the esterification. Feedback?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The possible issue is likely to be the decarboxylation at the ClCOCH2COOH stage. This intermediate should decarboxylate at the temperatures required
for the unavoidable fractionation (55-100 °C). Since acid chlorination with acid chlorides is highly reversible you always have the sensitive
intermediate present all the time in the mixture in considerable amounts:
2BzCl + CH2(COOH)2 <-> BzCl + BzOH + ClCOCH2COOH <-> 2BzOH + CH2(COCl)2
This is very much unlike in the irreversible chlorinations performed with SOCl2 or oxalyl chloride, where decarboxylation would not be much of an
issue. Since the reaction with BzCl relies on the removal of the lowest boiling acid chloride from the reaction equilibrium, the actual product is
likely to be either acetyl chloride or its mixture with malonyl chloride. In any case, the answer can only be found by doing a literature search or
experimentally.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
| Pages:
1
2 |