Nernst
Harmless

Posts: 13
Registered: 18-9-2008
Member Is Offline
Mood: No Mood
|
|
Synthesis of Tartrazine and Allura Red AC
Hello,
as a short member but long viewer of this genious forum, I'm pretty sure I'm finaly gonna get my answers.
For a school project I'm looking for 2 synthesis. I've searched freepatens & orgsyn already, but with no luck.
So, now I'm here with the big guys. Could someone provide me the synthesis of tartrazine &Allura REd AC?
Tartrazine (E102):
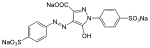
Allura Red AC (E129):
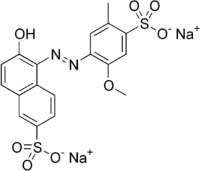
ps, Sorry for my bad english, normaly I speak dutch.
ps2: Jor or Woelen, do you guys know what's going on with our chemieforum.nl?
Edit by Texium: changed title for clarity/searchability
[Edited on 5-15-2018 by Texium (zts16)]
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Nernst,
According to woelen, the chemieforum is now 5 years old, and probably the admins have forgotten to pay the 'bill' for the domain. Probably they will
pay the following days, bringing the site back online. If they don't within a month, we might have a problem! Then it would be possible for anyone to
take over the domain www.chemieforum.nl! 
|
|
|
Nernst
Harmless

Posts: 13
Registered: 18-9-2008
Member Is Offline
Mood: No Mood
|
|
Oh, they haven't payed their bill. I've read on the site that the site was going to another server...
Now, for the synthesisis. Does anyone have a suggestion where to look?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
EIDt: Got ya!
| Quote: | | "FD&C Yellow N° 5 (Tartrazine, color index N° 19140) is a monoazo dye having pyrazoline ring structure. It is synthesized by condensing
phenylhydrazine-p-sulfonic acid with oxalacetic ester. This reaction product is then coupled with diazotized sulfanilic acid. The resulting ester is
then hydrolyzed with sodium hydroxide. Alternatively, tartrazine can also be synthesized by condensing two moles of phenylhydrazine-p-sulfonic acid
with 1 mole of dihydroxytartaric acid. FD&C Yellow N° 5 is an orange yellow powder. It is readily soluble in water, yielding golden yellow
solutions." |
From the Handbook of Food toxicity.
Looks more simple than what I would have though! I guess you need to wok your way to phenylhydrazine-p-sulfonic acid.. Which I think can be made my
reacting diazotized p-sulfanilic acid with sodium sulfite.
| Quote: | | "FD&C Red N° 4, (Rouge Ponceau SX, color index N° 14700) was approved for food use in 1929. This monoazo dye is synthesized by coupling one mole
each of diazotized 1-amino-2,4-dimethylbenzene-sulfonic acid and 1-naphtol-4-sulfonic acid. A red-colored powder, it ios readily soluble in water,
yielding orange-red solutions." |
Food Additive Toxicology
PS: you should look in the site library at the dye chemistry books availble. I'm sure you will find further info over there, especially on the
preparation of the precursors an general process. There also lots of info in the google books!
[Edited on 5-10-2008 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Nernst
Harmless

Posts: 13
Registered: 18-9-2008
Member Is Offline
Mood: No Mood
|
|
Klute, you're the best! I think I'm gonna have a nice coffee and read, read, read....
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Glad I could help! If you perform the synthesisfor your project, please report your results back here!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Please, take pictures too!
|
|
|
Nernst
Harmless

Posts: 13
Registered: 18-9-2008
Member Is Offline
Mood: No Mood
|
|
I would love to report my results en post the procedure with pictures but I'm stuck with finding the synthesis.
The only thing I can find is:
Tartrazine production involves two stages: (1) the condensation of phenylhydrazine p-sulfonic acid with sodium ethyl oxalacetate and (2) coupling the
product with diazotized sulfanilic acid.”
Stage 1:

By nernst
Stage 2:
Production of the diazotized sulfanilic acid.

By nernst
Tartrazine is produced by the reaction of the condensation product with the diazotized sulfanilic acid:

By nernst
This reaction is based on the methylene reactivity of the pyrazolone ring. Reactivity is observed because of the position of methylene between two
carbonyl groups in 1-(p-sulfophenyl) pyrazolone-3-carboxylic acid compound.
[bewerken aan 7-10-2008 door Nernst]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Well, you have it, no?
The reaction is detailed on page 265 of this book:
http://www.sciencemadness.org/library/books/fundamental_proc...
I urge you to read this book! And the others on thsi subject in the Sciencemadness Library! You have all the preapration of most of the precursors in
there, and the reaction conditions for the condensations.
Sulfanilic is an cheap commercial product, or can be made from aniline by basic reactions. The hydrazine is formed by reacting the diazotized aniline
with sodium sulfite (p. 128 of the book cite dabove):
Ethyl oxalacetate can be prepared by a variety of methods (although it can be availble commercialy):
-Claisen between diethyl oxalate and ethyl acetate:
http://www.freepatentsonline.com/4902819.html
http://www.pat2pdf.org/patents/pat1948201.pdf
-acylating acetoacetate with mono ethyl oxalyl chloride (EtOOC-COCl) or a similar ester, selectively de-acetylate, then hydrolyzing the oxalate
ester:
http://www.orgsyn.org/orgsyn/pdfs/CV3P0379.pdf
http://www.orgsyn.org/orgsyn/pdfs/CV2P0266.pdf
http://www.orgsyn.org/orgsyn/pdfs/CV4P0415.pdf
-acylating diethyl malonate with the same ester, and hydrolyzing the oxalate ester after mono-decarboxylation:
http://www.orgsyn.org/orgsyn/pdfs/CV4P0285.pdf
All the rest in described in the Dye books.
the reactivity of the emthylen group issimply du to it being in alpha position of the "hydrazine amide", not the the carboxylate which is two carbons
away.
I think that's all I can do for you, can't do the reactions myself  . the info is
just right one the first page on this site . the info is
just right one the first page on this site 
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|