| Pages:
1
2
3
4
..
24 |
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I've collected everything that I need to perform the reaction of urea with nickel powder to produce hydrazine. All new glassware (50 ml 24/40 RB
sure looks sized odd), good big stir bar, nice new hotplate, liebig condenser, etc... I'm going to wrap the whole apparatus in two or three
layers of aluminum screening in case of detonation for some reason or another.
However I'm wondering about the exit gasses, CO probably mixed with some Ni(CO)4, my current plan is to run them into a Bunsen burner and subject
them to high temp, burn the CO and the Ni(CO)4. But does anyone know of a solution I could run them into to neutralize them, seeing as how that would
be an easier alternative. I know that a NaOH solution will react with CO but I believe it's only at elevated pressure.
One other thing, Marvin you said you have paper copies of both patents, I only know of the one patent, what's the patent number for the other?
And one last thing, I was considering running the reaction in a solvent. Just preliminary solubility queries yield urea to be soluble in benzene and
hydrazine to be pretty much insoluble. So with efficient stirring the hydrazine should sink to the bottom and the nickel will be in better contact
with the urea in solution, also the carbonyl will be soluble in the benzene so it might help to moderate the reaction. But I've got a bad habit
of trying to modify reactions that I've never run so I'm going to run it by the book a time or three but the solvent sounds like a
reasonable modification to me.
[Edited on 3/7/2004 by BromicAcid]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Urea is nearly insoluble in ether and chloroform. My guess is that it wouldn't disolve well in benzene. I can't find a lot of solubillity
data on hydrazine but it is soluble in the lower alcohols and in water. I would be a little suprised if it isn't reasonably soluble in benzene.
(Ammonia is, ethylene diamine is too).
I would also be wary of using benzene as a solvent if I could avoid it, on the grounds of toxicity. Then again, I don't supose you will be
sniffing the reaction mixture.
IIRC you can use solutions of copper (I) compounds in ammonia to trap CO. NaOH won't do the job under normal conditions (About a million years
ago I did a university practical investigating the CO/CO2 +C equilibrium. The CO was measured over NaOH soln)
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I don't know how soluble it is but under the entry for urea it is listed as being soluble in benzene. The main reason I was considering a
solvent is that looking though the patent it states that at low temperature reaction 40 - 60 C a large quantity of nickel carbonyl vapor comes over.
I figure with an organic solvent not only will the nickel carbonyl be absorbed and hopefully have more time to decompose before leaving the solution,
but it will afford better contact between the nickel catalyst and the urea.
So I'm looking for a solvent with the following properties:
1) Urea is atleast slightly soluble in it
2) Nickel carbonyl is soluble (it's soluble in most organics anyway)
3) Bp >60C but, <100C unless, >135C
4) Does not form azetrope with NH2NH2
5) Will not react with hydrazine, finely divided nickel, nickel carbonyl, urea, or carbon monoxide
By the way, does anyone have solubilites for nickel carbonyl in any solvent besides water?
[Edited on 3/7/2004 by BromicAcid]
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
You do not want to increase the residance time of the nickel carbonyl in the reaction mixture. It wont decompose at the low temperatures so the
reaction proceeds forwards mainly by loss of nickel carbonyl (the reason nickel needs to be in excess)
This reaction is reversable, that is to say CO and hydrazine will combine in the presence of nickel metal to form urea (mislaid my reference for this,
but given the nickel process for hydrazine production, I didnt need much convicing that it was reversable). More of a pain is that hydrazine left in
the reaction mixture will react with urea to form semicarbazide, which constitutes a loss if you only want hydrazine. You can form urea/semicarbazide
in the reciver if you use iron salts/other metals as explained in the patent.
Hydrazine hydrate attacks glass, but Ive been told dry hydrazine does not. So long as the mixture is fairly water free, this might not be a problem
for you. A more worrying rumor is that ground glass joints can cause hydrazine vapour to ignite/detonate. I dont remeber where I read this, or if
this is accurate.
If you are going to try the process, I would avoid the low temperature method producing large amounts of the nickel carbonyl and use the temperature
neer the decomposition point of urea yeilding almost entirly carbon monoxide, and condensing the hydrazine out of this.
Call me daft, but I think it might be preferable to mix the CO with the fuel of a gas burner, flush the system continually with say butane and burn
the offgas. Pass the gas through the cold setup in order to exhaust the oxygen for some time before igniting it (you know this). Butane should be
resonably inert to the reaction.
Other patent number is 2675301. It seems almost identical. Its actually mentioned in the later paper.
unionised, remeber that ten thousand factor I mentioned earlier? I really dont think benzene would make the slightest difference to the overall risk.
I do think it would be detrimental to the experiment though.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Marvin, I was unaware that hydrazine and carbon monoxide in the presence of nickel could form urea, thanks a lot, you saved me on that one. I read
that other patent, I missed it in the first place because I copied the other patent onto a wordpad document by hand and just ignored the header.
Supposedly according to it the reaction of urea with hydrazine is minimized at atmospheric pressure and nickel carbonyl losses are significantly less
at elevated temperatures. Oh well, I guess I'll run this one in an oil bath.
I was somewhat worried about the explosive decomposition of hydrazine on ground glass joints, supposedly the greatly increased surface area can cause
it to catalytically decompose. I'm going to go heavy on the silicon grease and hope for the best, it seems to be a rare phenomenon anyway.
Also I was reading up on hydrazine as a mono-propellent in rockets. It is decomposed by and Ir-Pt catalyst, or a silver screen catalyst, or a
tungsten oxide catalyst, or an Ir-Al2O3 catalyst. No mention of a nickel catalyst which was one thing that I was worried about, expecially after
seeing the kind of heat and energy generated.
So I guess a solvent is not the way to go on this one, heat and hope for the best, ultra-clean glassware, greased joints, exit gasses run into the
intake on a bunsen burner. Wrap in screen as a last ditch save attempt. Magnetic stirring, I think I've got this trial run all figured out. I
think I'll go for a charge of 25 g of urea and 12.5 g nickel, bearing in mind that 50% nickel by weight gave the best yields in the patent.
[Edited on 3/8/2004 by BromicAcid]
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Hydrazine is usually distilled on a lab scale in silver retorts. In so much as I had any 'good' ideas when reading this stuff, I wondered
about protecting glassware with the silver mirror reaction. It would probably flake off neer joints, but it could help the glassware from cosmetic
damage, and also a 'frosted' effect that might catalyse decomposition.
Probably not something youd want to try on a first run, but I think I'm better mentioning it than not.
I see you seem to be avoiding my idea of running the fuel through the equipment and out into the burner in favour of feeding the CO into the air
intake of the bunson. As long as you purge the container first I dont see a problem. Other than the toxic compounds, the possability of hydrazine
explosions and flying glass that is. Mmm. Did I mention how glad I am that I'm over here and your all the way over there. I'm sure I did.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
| Quote: |
I see you seem to be avoiding my idea of running the fuel through the equipment and out into the burner in favour of feeding the CO into the air
intake of the bunson. As long as you purge the container first I dont see a problem.
|
Well I didn't want to run fuel through it for two minor worries. One, the propane or whatever lower hydrocarbon I put through the system may
well be soluble in hydrazine and contaminate the product to a high degree. And two, running the fuel through the system may increase the pressure
because it will be running through the small bunsen burner hole and an increase in pressure in this reaction is associated with a loss of yields.
Both are minor reasons but still things that I've been thinking about. Also, can't hydrazine decompose catalytically without oxygen if the
temperature is fairly high. E.g. a flame could flash through the equiptment from where the gasses are being burned off and detonate everything
regardless of it being purged of air? If so, maybe I should run the exit gasses through some bath first to prevent it from flashing back into the
system.
| Quote: |
Did I mention how glad I am that I'm over here and your all the way over there. I'm sure I did.
|
Not directly but it has been implied. Based on your warnings I have decided to scale down my reaction vessel. Going to run it in 14/20 glassware if
I can get one more piece. Wash all the glass with alcoholic NaOH beforehand, oven dry, assemble quickly. Wear proper protection, long gloves, face
shield. I have all the equiptment I need to do this safely. But I still won't be doing this for a month or so.
[Edited on 3/9/2004 by BromicAcid]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Smaller scale first?
I wonder if the form of the nickel powder is especially important to its reactivity. Will nickel powder formed from zinc dust and aqueous NiCl2
solution work, or do you need to prepare nickel formate and decompose it with careful heating while protected from air?
Before you even attempt isolating the hydrazine, why not try this on a test tube scale? Urea decomposes fairly readily with heat anyway, so bubbles
aren't the sure mark of success. It would be nice to first verify that you're producing some hydrazine before you scale up. Will hydrazine
decolorize iodine starch test paper? I don't have a qualitative test handy, but I'm sure you can look one up.
Also, second idea: assuming that it works on a test-tube scale, how about passing your exit gases through aqueous H2SO4 when you first scale up?
Hydrazine sulfate's useful, and it's a conveniently weighable form (for judging yields), and it's far less hazardous. "Walk before
you run" as the saying goes.
PGP Key and corresponding e-mail address
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I'm going to be running a test tube scale first. I got some flint glass and made it into the shape I needed so that it would exit the
'hydrazine production tube' and go a distance then down into some cold 6M H2SO4.
I've got everything I need but some nickel powder. According to the patents nickel of any shape or form can be used. From the powder used in
the small scale to the sizable balls of nickel that would be used in the continuous process mentioned where molten urea is constantly circulated
through a bed of Ni. I made some NiCl2 but I need to reduce it somehow. It's still slightly acidic and in solution and I'm trying to
reduce it without evaporating all the choking fumes then reconstituting then reducing. I tried Al with no success, zinc made a white precipitate,
odd. Sn in HCl made small amounts of Ni powder but nothing sizable, let it go for two hours, maybe next time longer. Any easy reducing agents anyone
can think of?
Regardless next weekend is the next time I will be experimenting so that is when I will attempt this process, if I can't get my Ni powder by then
I will just go with nickel chips.
[Edited on 4/3/2004 by BromicAcid]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Zinc should have worked. I had no trouble producing nickel dust from zinc dust swirled in acidic aqueous NiCl2. However, my crude "nickel
powder" had a considerable amount of zinc left in it. This I was able to remove by treatment with dilute acetic acid (which dissolved the zinc
much more readily than the nickel), leaving me with a fine black dust.
PGP Key and corresponding e-mail address
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I made an attempt today.
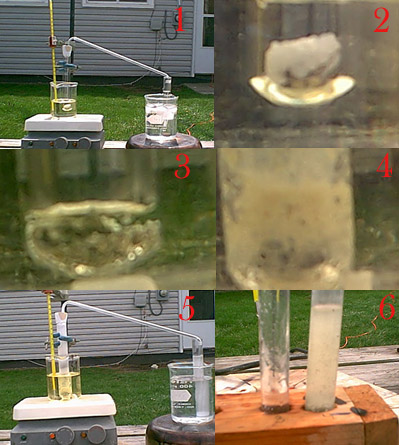
This is the apparatus prior to any heating or anything. The test tube has been charged with about 3 g of urea and 2 g of nickel in the form of
filings and small pieces also a stir bar was thrown in. This was in an oil bath that also contained a stir bar to allow for evener heating. The
thermometer is also placed in there. The exit gasses passed through a piece of flint glass and into a second test tube in an ice bath filled with 6M
H2SO4. Immediately after this picture was taken heating was begun.
Close up on the contents of the test tube prior to heating.
The mixture melted at about 137 C and heating was slowed and it stayed relatively constant at this temp. I held it here for about 20 minutes
with very very infrequent bubbles. No precipitate came in the sulfuric acid so heating was increased after this time period.
Over the course of one hour heating gradually increased. This picture was taken when the mixture had reached 200C. It was constantly bubbling
and expanding from the frothing. At the lower part of the test tube there was condensation that hardened shortly above the oil line. Then there was
a space with no condensation and right at the top it was foggy due to additional condensation. Probably just urea. Meanwhile in the other test tube
there was no precipitate. However a crust would occasionally form right were the gasses exited into solution and would break off in a semi-circle and
float to the top so the top looked like it was covered in a thin skin, hydrazine sulfate?
It was shortly after the last picture that heating was discontinued entirely. Notice the foggy condensation on the generation test tube. The
second test tube is somewhat milky white. This occurred because it got so cold after the H2SO4 addition. It cleared up after I let it sit awhile.
Although there was a crust on the top the picture that I took did not turn out.
For the test tube that collected the gasses I added copper sulfate as mentioned further up thread as a test for hydrazine. I got a weird thick
skin on the top of the liquid after stirring that looked remarkably like ice. Other then that I had no additional tests. I took the other test tube
that had generated the gasses and added water to put some of it into solution. It made a milky white precipitate filled liquid. Copper sulfate
produced no effect.

To me it looks like it didn't work too well if at all. Gas evolution was only significant at about 160C below that bubbles were few and far
between. The nickel came out blackened. A good sign that something was going on with it but other then that nothing else. I plan on running this
again next weekend with iron in place of nickel. And if that even doesn't work one last time with 14/20 lab ware with about 20g of urea, seeing
how sluggish the reaction was with nickel shavings and such I believe the next attempt will be completely nickel powder. But as it looks now I
don't think barely any hydrazine was formed at all.
[Edit] One other thing, the molten urea turned green towards the end, is that normal? I thought that maybe some nickel carbonyl may have formed and
by all accounts I would consider molten urea an organic solvent so it could have went into solution and caused the color change.
[Edited on 4/11/2004 by BromicAcid]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I have a feeling that molten urea would disolve nickel oxides and give a green solution. It might well disolve nickel in the presence of air.
The decomposition of urea without a catalyst is a complex affair. How do you propose to prove the presence of hydrazine rather than biuret, cyanic
acid, melamine or whatever?
BTW, is anyone in a position to check out this reference?
http://www.maik.rssi.ru/abstract/rjapchem/97/rjapchem1067_ab...
[Edited on 12-4-2004 by unionised]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Unionised: You cannot see that page? All I can see is the abstract, is this the same for you or can you not see the entire document. Anyway here is
the abstractif you cannot see it.
Quote:
<Quote>: Abstract: The catalytic decomposition of hydrazine is studied with regard to the needs of the airspace industry. Data are presentsed
on the rate, stoichiometry, and activation energy of hydrazine decomposition on metals of platinum and iron groups and also tungsten and molybdenum,
supported by alumina. the effect of ammonia and water concentrations on the rate of hydrazine decomposition is studied. the difference is
demonstrated in the rates of liquid-phase and gas-phase decomposition of hydrazine. Phenomena are discussedrelated to the transition from
heterogeneous decomposition of hydrazine to the homogenous process.</quote>
[Edited on 12-4-2004 by rogue chemist]
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
| Quote: |
The decomposition of urea without a catalyst is a complex affair. How do you propose to prove the presence of hydrazine rather than biuret, cyanic
acid, melamine or whatever?
|
Being that the solution was hot enough to volitize any hydrazine formed I lead it through a 6M H2SO4 solution. If any hydrazine was formed in any
sizeable quantity I was relying on it reacting with the sulfuric acid and forming the firmilar hydrazine sulfate precipitate. Then I could have done
some tests on the precipitate, e.g. saturated a hot solution with it then added CuSO4 like mentioned up thread to see if I got a precipitate from
that.
Also, if I didn't go for that and just tried to collect a distilate I could have tried to dissolve sodium metal in the distillate and if it went
in with no reaction other then color change then I could have reasonably assumed (considering the distillate would be at stp) that it was hydrazine.
Looking over the evidence again I really don't think the reaction would go well. The carbon really doesn't resemble the carbon in carbon
monoxide at all so I would assume it would not as easily form carbonyl compounds, it is lacking the electrons to easily form them. However at the
higher temperatures with decomposition occuring anyways the carbonyl may well form. Next run, greater quantities, straight nickel powder, longer
reaction run.
Maybe I could run it in aqueous solution in the presence of an acid like HCl at about 80C. This would protonate the nitrogen and give it a positve
charge which would draw some of the electron density from the carbon and possibly make it more reactive. Just a hope but oh well, time for some
experimentation.
[Edited on 4/12/2004 by BromicAcid]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I could see the abstract; I wanted to know what the full report said. In particular, I wanted to know if nickel was among the platinum group metals
that decompose hydrazine. If it is then this method is dead.
In the presence of enough HCl to protonate the urea, the metal catalyst will disolve and the urea will hydrolyse.
From 3 grams of urea, if you got 100% yield you would get about 1.5 g of hydrazine which would give you about 6.5 g of the sulphate.
That would dissolve in about 200 ml of water. If you have about 20ml of dilute acid in which to trap the product then you need at least a 10% yield to
get any crystals of hydrazine sulphate.
The formation of crystals is not a sensitive, or specific test.
[Edited on 13-4-2004 by unionised]
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
hydrazine reaction alternative chelator
What chelators can be used instead of EDTA for hydrazine synthesis by NH3 and NaClO?
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Cysteine (which is found in egg whites) could probably be used. I don't have the references handy as to which metal ions are the most
detrimental to hydrazine formation (I think they all are to some extent) but the necessity of a chelator can be lessened by using something to
'gum up' the mixture as most synthesis on the web say add some gelatin. However your yeilds from the partial oxidation of ammonia are going
to be low by definition due to the side reactions.
[Edited on 5/2/2004 by BromicAcid]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Does this mean one can use the "slime" from those kiddie chem sets? 
Also BromicAcid here is a hydrazine test kit. http://cgi.ebay.ca/ws/eBayISAPI.dll?ViewItem&category=20...
[Edited on 2-5-2004 by rogue chemist]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Hydrazine Regurgitation
heskogen was asking some things in another thread, answers that were in other threads, like this one. This got me looking into my notes for additions
here. This contains marginally useful factoids but no syntheses, so turn away or don't. This is mostly well-known (I thought) stuff, intended for
the reading-impaired, in case it can ever be found useful. So some of it has been mentioned already. Some of it is just stuff I wrote down and
don't know why. Feel free to say its not all practical.
First of all, www.orgsyn.org. CV 1, 309. Go there. There are references to patents, etc. in addition to the bad-yielding hydrazine prep. Next, ep.espacenet.com.
The original patents by Rachig: DE192783, DE198307, US910858. His article: Ber., 40, 4580 (1907). Libraries! Microfilm! Also, GB199750.
Explanation for heskogen/the unaware: The reaction NaOCl + NH3 = NH2Cl + NaOH is very easy even cold. The reaction NH2Cl + NH3 + NaOH = N2H4 + NaCl
+ H2O is not so easy as it requires heating to boiling or close to it. Much easier is the reaction 2NH2Cl + N2H4 = 2NH4Cl + N2. There is another bad
reaction: 3NH2Cl + 3 NaOH = 3NaCl + N2 + NH3 + 3H2O. So its kind of important to get the chloramine reacting with NH3 instead of something else.
Some experimental with molar excess NH3/yield based on NaOCl:
6X/25%, 10X/38%, 20X/50%, 37X/63%, 77X/75%.
Rachig found that an additive was needed. Large amounts of glycerol, sugars, and starch increased yield to 40%. Albumin, casein, and glue gave 60%
Glue and a large excess of NH3 finally gave 75%. Some experimenting with Knox gelatin (typical American brand), all using 200 ml. 28% NH4OH and 100
ml. 7.5% NaOCl, in wt. of gelatin in mg.'s/yield N2H4:
0/1%, 3/20%, 10/44%, 50/63%, 100/71%, 500/77%, 1000/88%, 2000/92%, 3000/91%, 4000/91.5%
Some quantitative tests for hydrazine, months late:
N2H4 + HCl + KIO3 = KCl + ICl + N2 + 3H2O. The iodate is added until the iodine color goes away. The HCl conc. is such that it is 3-5N at the end
point.
Alkaline KMnO4 gives MnO2, alkaline ferricyanide gives ferrocyanide.
A neutral solution that can contain a very small quantity of N2H4 gives a precipitate of the azine when shaken with benzaldehyde (straight out of the
imitation almond bottle?), propionaldehyde, or salicylaldehyde. The precipitate dissolves, however, in excess aldehyde. This is not necessarily bad,
if you have a lot of aldehyde that can act as reagent and water insoluble solvent. These azines are even less soluble in cold H2O than benzaldehyde is
in warm. There is a synthesis of methylhydrazine via this route in Org Syn CV 2, 395. The aldehyde can be regenerated by acid hydrolysis.
Some qualitative tests: Fehlings gives Cu. Acid chromate gives Cr+3. Of course peroxide, persulfate, chlorate gives HN3.
Allegedly an interesting way to plate glass with Cu: 2g. cupric acetate and 100 ml H20 are mixed with enough NH4OH to dissolve the precipitate. 15 ml.
40% hydrazine hydrate is added and all is dripped onto a glass object, at 60C, until a shiny layer of Cu plates the glass. The glass is washed with
hot water and put in a bucket of 60C water that is allowed to cool at its own pace.
Similar things can be done with silver (GB524753) and nickel (DE717547) also Pt and Au. This Ni plating may or may not be helpful with procedures
that call for 10% KOH in alcohol and other caustic reactions. Even plastic can be plated from hydrazine and ammoniacal Ag nitrate. I would think that
this may be useful for preparing supported catalysts. US1164141 uses this to make a non-Raney Ni catalyst.
Saw acetone mentioned. I read somewhere that the ketazine/acetone/water distillate can be acidified with H2SO4, giving the sulfate and acetone, ref:
JACS 51, 3394 (1929). Of course the ketazine can be extracted with organic solvent instead.
Saw urea mentioned. I also have here that the max yield with this in any lab is 60%.
Hydrazine sulfate solubility in water, in deg C/g. per 100 ml H20:
20/2.8, 40/4, 60/8.3, 80/12.6
And at 25C in H2SO4, in g.H2SO4 per L/g.N2H4H2SO4 in 100g. solution:
.5/3, 5/2.7, 26.6/1.5, 49/1, 116/.5
Notes on drying: hydrazine hydrate can be refluxed with an equal wt. of NaOH for several hrs, then distilled at normal pressure to give anhydrous
hydrazine. If the reflux is skipped the product is 90-95% hydrazine. If not skipped, a temp of 150 is necessary even though the bp is 113.5C.
85% hydrazine hydrate can be dehydrated by heating with an amount of NaOH equivalent to the H2O it has. KOH cannot substitute. 2 layers separate on
heating and the layers contain in % by wt.:
C---N2H4--NaOH--H20 -upper layer:
60--77.5-----9.3----13
70--91--------3-------6
90--92--------2-------6
lower layer:
60--19------51.4---29.5
70---6.7----67.6---25.5
90---6------69.3---25
-from JACS 71, 1644 (1949)
The original method for anhydrous hydrazine: NaOCH3 in MeOH + N2H4HCl = NaCl + CH3OH + N2H4. The alcohol is distilled off, and you must really want
anhydrous hydrazine if you abuse your Na in this fashion.
Dilute hydrazine solution has been concentrated by hot (over 50C) anhydrous NaSO4, with the decahydrate crystallizing on cooling. Some water and
some sulfate will remain though.
Xylene has been mentioned for azeotropic drying of dilute solutions, and Vogel recommends this to obtain 90-95% hydrazine hydrate. Toluene should
work, though not as well.
Last factoid: H2SO4 precipitates, at best, only 90% of the hydrazine in dilute solution.
I'm sure that none of this is true and all the important parts have been left out. You know how books are!
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Today is the day, I've completed a fair bit already and here is the step by step:
Placed 8 g of nickel oxylate dihydrate into a 50 ml glass volumetric and stoppered loosely with a 10/30 stopper. Also a very small amount of
HgO (<.0005 g) was added, this has a catalytic effect of promoting nickel carbonyl formation.
Heated slowly on a hot plate, first lots of water came off, I took the stopper out to just let the water evaprate away then it started to turn
black at the bottom so I re-stoppered it. It continued to release gas until the whole mass turned black. Upon swirling the hot flask the power was
free flowing. I set aside and allowed to cool.
I esitimated that my reaction should have yeilded slightly over 2 g of nickel power so while it cooled a little bit I weighed out 6 g of urea
and placed that along with a stir bar in a 100 ml 14/20 RB flask.
All parts were previously washed and washed again with distilled water. They were dried in an oven and the joints were wrapped with teflon
tape.
I added the cooled nickel powder to the urea flask, it formed little needles on the stir bar. I quickly put the still head in place and the
rest of the assembly, a gas entry tube was put into the thermometer hole on top.
H2S (which supposedly increases the activity of nickel toward forming the carbonyl) was slowly put into the system, a very small amount total.
The gas entry tube was replaced with a stopper and the apparatus was lowered into an oil bath and heating begun.
The mixture liquified and the urea took on a blue tint, probably some nickel oxylate that didn't decompose.
The mixture is currently heating away as it has for almost an hour. It is being held at 150 - 160 C and urea is crystalizing on the inside of
some the glassware near where the reaction flask is.
There is no distillate so far, but I will keep posted. If I get distillate I will attempt to dissolve sodium metal in it. If it is anything but
liquid ammonia (impossible) or hydrazine it should react appreciably.
Edit: Ran the reaction for 1 hour 30 minutes, no distillate, tiny little hairs of urea condensed on the inside of the reaction flask though. Urea
crystalized though 80% of the glassware, no liquid anywhere. Heavy ammonia smell even though exit gasses were being washed.
Then I turned up the heat to 200C for 15 minutes, urea decomposed more but no sign of a distillate. 
I think I'm done trying this for a while until someone else shows some promising results. If freshly made nickel powder will not react with urea
with every accomidation made for the formation of carbonyl, I give up.
[Edited on 9/27/2004 by BromicAcid]
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Hydrazine reacts with Na to form NaN2H3
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I've read in a few places that the solvated electron trick can be done with hydrazine as well as liquid ammonia to yeild the characterisic blue
solution.
However I'm sure the reaction takes place, similar to the anologous reaction between ammonia and sodium to produce sodium amide, which is
catalyzed by trace metal ions and moisture.
Edit: Although.... after looking around a bit I realize this might not be the best idea to test for hydrazine....
| Quote: | | Alkali metal hydrazindes, formed by dissolving the metals into hydrazine, are one of the few substances which behave as bases. These tend to be
pyrophoric with air, and sodium hydrazide (NaN2H3), for example, explodes violently when heated above 100 C. As a result, alkali metals are
incompatable with hydrazine. Alkali metals are common to reduce melting temperatures in some glasses-glass composites, for example.
|
From Here
And....
| Quote: | | Anhydrous hydrazine and sodium in ether react to form sodium hydrazine which explodes on contact in air.... |
From Here
So yeah, maybe it's a good thing I didn't get a yield.
[Edited on 9/27/2004 by BromicAcid]
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Immediately after the reaction mixture cooled after tying this synthesis I took it and cleaned it. But there was an adherent layer all in the
reaction flask and going up into the still head that I could not clean off. I decided to soak it in concentrated HNO3 for a week or so.
Today when I pulled it out it was obvious what the gunk on the glassware was. Something had ate it somewhat terribly.
Hydrazine was made, not much but some. Maybe someone else with more patience then me can one day achieve a decent yield by this reaction.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Hydrazine can also be made by reducing nitramine. Nitramine is made by hydrolysis of dinitrourea, that being made by dehydration of urea dinitrate,
that is made by reacting urea and nitric acid (ask your buddies at E&W  ).
The reducing agent could be sodium sulfide or something. ).
The reducing agent could be sodium sulfide or something.
|
|
|
Mongo Blongo
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
I can't seem to find any information on Urea Dinitrate. Does it form with concentrated HNO3 rather than just Urea Nitrate at lower
concentrations? I assume it can be precipitated with water right?
|
|
|
| Pages:
1
2
3
4
..
24 |