mechem
Harmless

Posts: 32
Registered: 27-12-2007
Member Is Offline
Mood: No Mood
|
|
300 deg/C oil bath
Hi
Does anyone have any ideas on what substance/liquid could be used in an oil bath, maintained at 300deg/C, without smoking the place out.
Thanks
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
-40 to 250° (to 400C under N2)...... D.C. 550 silicone fluid
Up to 340°......... A mixture of 85% orthophosphoric acid (2 parts) and metaphosphoric acid (1 part)
60 to 500C............ Fisher bath wax (highly unsaturated)
73 to 350°.......... Woods Metal*
250 to 800C........ Solder*
* In using metal baths, the container (usually a metal crucible) should be removed while the metal is still molten.
(Source: Armarego, Purification of Laboratory Chemicals. 5th Edition, P35)
Also:
"A fused salt bath consisting of 8.5 parts (by weight) of sodium nitrite and 10 parts of potassium nitrate has a melting point of about 140° and may
replace the [Wood's] metal bath." Source.
[Edited on 7-10-2008 by sonogashira]
|
|
|
jokull
National Hazard
   
Posts: 506
Registered: 22-2-2006
Location: Everywhere
Member Is Offline
Mood: Ice glassed
|
|
You may use:
Paraffin
Melting point 50º C
Useful range 60-300º C
It is flammable
Dibutyl o-phthalate
Melting point -35º C
Useful range 150-320º C
It is generally used
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Dibutyl phthalate is available in 4L containers and has a published boiling point of 340*C. Does it smoke before it gets there? I don't much care
for boiling parafin. I'm using silicone brake fluid for up to about 200 but it starts outgassing before. Not sure how it will behave if I run it up
higher. Might be something to try and report. Phthalates are pretty cheap although they represent something of an environmental problem. One of my
professors had us using hydrolized cottonseed oil and everyone on the floor got a headache. That wasn't even for high temps. I don't care for any
kind of waxy material as too hard to clean up.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
mechem
Harmless

Posts: 32
Registered: 27-12-2007
Member Is Offline
Mood: No Mood
|
|
Forgot to mention I need a bath with about 3 to 4 liters of fluid, expense is also an issue otherwise solder or molten metal alloys would be my first
chose. KNO3 with NaNO2 sounds interesting but getting hold of NaNO2 might be a problem, any other combinations.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Brake fluid started smoking (nasty) at around 175*C. I'm for trying pthalate esters.
[Edited on 7-10-2008 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
It's probably completely unhealthy to inhale paraffin-smoke ! There's also the wisdom amongst smokers: Not to light a cigarette from a candle;
probably because of the unburned paraffine when sucking it through the tobacco, where a small dosage will cool to below the flamepoint.
With vacuum-pumps there is also the issue of not letting the oil-dust (exhalation) get into the lungs, probably it's the same with paraffine. Maybe
the body can't get rid of it and it devalues the breathing-effectiveness ...
|
|
|
tapira1
Hazard to Others
  
Posts: 168
Registered: 9-10-2006
Location: Here!!!
Member Is Offline
Mood: 
|
|
hightemp bath
Use a sand bath; it is clean, cheap and safe. It performs as the best liquid bath.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
The problem with a sand bath or woods metal bath for that matter is you have to heat it with flame. I know the grand old masters heated ether with
flames but I like to avoid flame in my lab. The porosity of sand makes it too expensive to heat sand with a hot plate. If you can get one hot enough
that is..
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemrox
The problem with a sand bath or woods metal bath for that matter is you have to heat it with flame. [...] The porosity of sand makes it too expensive
to heat sand with a hot plate. If you can get one hot enough that is.. |
Why the felt need to use a hot
plate? Surely an immersed mantle inside the sand, with the sand held in an insulated container, works just fine, and is just as electrical as a hot
plate. For that matter, why not take apart the hot plate and use its element as the mantle?
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Like watson.fawkes said, i made my own mantle by attaching a kettle heating element to the bottom of a metal bowl then filling with fine sand. This is
good for refluxing but it can get amazingly hot and is hard to control the temp.
I have used an electric hotplate to heat a sand bath with no probs. Just wrap Al foil around it to keep some of the heat in and its fine.
Remember, the finer the sand the better.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | | Originally posted by chiefThere's also the wisdom amongst smokers: Not to light a cigarette from a candle; probably because of the unburned
paraffine when sucking it through the tobacco, where a small dosage will cool to below the flamepoint. |
sorry, this is totally off topic, but i just had to say it: HA HA HA! does it really matter if you get that small amount of soot from a candle in your
lungs when you smoke a cigarette? 
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Fair point! your lungs are mucked up anyway! a little parrafin wont change much! 
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
The lungs clean themselves regularly, and even formar-smokers can be healthy (some sort of) a whilelater,dependson the dosage. But there is still the
parallel with the oil-dust from compressors and vacuum-pumps, usual-safety-issue. And this meant oil-dust is too an mineral-oil-derivative ..
Anyhow I wouldn't subject myself ton any paraffine-smoke.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
COMPLETELY off topic:
"Not to light a cigarette from a candle"
I've heard that this is actually quite an old superstition. Apparently sailor's wives would place a lit candle in their windows when their husbands
were at sea, and lighting a cigarette off this candle would increase the chances of it going out, which would be seen as a bad omen for the sailor.
Which is why you will sometimes hear that lighting cigarettes of candles kills sailors.
|
|
|
DNA
Hazard to Others
  
Posts: 191
Registered: 11-6-2003
Location: @moon
Member Is Offline
Mood: Experimenting
|
|
I was just about the ask the same question (no not about the sigarettes), I've tried to do a ultra-micro boiling point determination.
I got very pissed of when the oil finally reached the 250*C with my hotplate, then it started smoking a bit and it wouldn't go higher.
The boiling point of the compound was around 260-270*C so that really sucked. Now I'm also indeed wondering what I could use better then the olive oil
from the supermarket.
I can get ahold of liquid paraffin oil and silicon oil, ultragrade 19 edwards vacuum pump oil (380*C boiling point  ) )
What oil is normally used in melting point apparatus?
DO MIND the autoignition temperature!!!
Just found that for the ultragrade 19 oil this is 355*C!
So the boiling point can nicely be 380 but when the oil will spontaneously catch flames is at 355 that is not really nice if you didn't expect that...
Santovac® 5 Diffusion Pump Oil
Has a boiling point of 476*C and autoignition of 590 so that one is even better, but probably also even more expensive than the ultragrade 19.
Another thing is glycerol/glycerine it has a boiling point of 290*C that is easy/cheap to get and has a nice boiling point...
Also found this:
[Edited on 9-10-2008 by DNA]
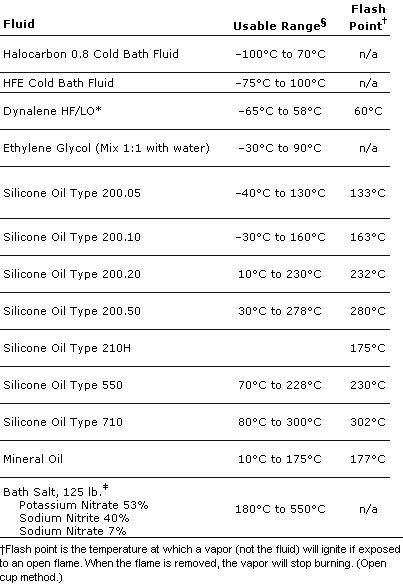
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
One other fact: The average livetime of professional cooks is limited to 60years only ! (in Germany), thats statistics. This means: For each one that
makes it to 70, 2 passoff at 55 ...
Maybe that comes partially from their oil-dust-breathing too ?
Besides: My version af oil-bath-substitute:
==> I have a hot-air-gun,
==> 2kW, max temp 630 [Cels] (but only 595 can be measured with a thermocouple),
==> temp digitally adjustable in 10 [Cels]-steps, from 50-630 [Cels]
==> and it coasted around 75 EUR
That thing just can be set to any temperature, and will hold it, even if the air-stream has to go through tight areas (higher streaming-resistance
==> less air will pass), due to the digital controller (which rapidly switches on-off the powerat the full 2 kW,net-consumption is less (eg.
half-temperature (300 [Cels] @ half air-volume nly 500 W ... ) nly 500 W ... )
[Edited on 9-10-2008 by chief]
[Edited on 9-10-2008 by chief]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I like the idea of building a sand bath from parts. One of you said the element he used was hard to control. I use a deep fat fryer as an oil bath
and the controller has been spot on to 1-2*C. The only name I can find on it is "Davey." I don't know if it would behave as well if it were filled
with sand instead of oil.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
mechem
Harmless

Posts: 32
Registered: 27-12-2007
Member Is Offline
Mood: No Mood
|
|
Does anyone have any experiences of heating Zinc chloride, which melts at 275-C and does not have the explosive drawbacks of KNO3 + NaNO2 if there was
a spillage. Mainly need to know if there is heavy fuming at its melting point, as I do not have a sample to test.
Thanks
|
|
|