| Pages:
1
..
3
4
5
6
7
..
28 |
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Here's a procedure which I took from Frogfot's old website which seems to now be down:
Introduction
Industrially benzoic acid is prepared by oxidising toluene with air oxygen in liquid
phase in presence of homogenous catalysts like salts of cobalt or manganese. On lab
scale it's more convenient to oxidise toluene with potassium permanganate.
Toluene can be found as solvent in most paint stores.
C6H5CH3 + 2KMnO4 ==> C6H5COOK + 2MnO2 + KOH + H2O
C6H5COOK(aq) + HCl(aq) ==> C6H5COOH(s) + KCl(aq)
Following procedure is taken from a book (see reference below), as you may notice,
they use lots of water, 350 ml, to prepare about 5 g benzoic acid, this is not good if
we wanna upscale procedure.. Do the math -- for 100 g benzoic acid we'd need at
least 7 litres jar.. Such big amounts of solvent are probably used to give reaction
mixture better agitation, otherwise, forming MnO2 will make reaction mixture too
thick. However, I found that there are no agitation problems with smaller solvent
volumes when using a magnetic stirrer, I've tested below experiment with only 100
ml water and achieved same yield as original procedure. Amount of water can be
probably decreased further (yet to test..).
Procedure
Materials:
Toluene (C6H5CH3)
Potassium permanganate (KMnO4)
Hydrochloric acid 30-40% (HCl)
In 500 ml round-bottomed flask equipped with condenser, prepare a mixture of 5,75
ml toluene, 350 ml water and 17 g fine potassium permanganate powder. Couple of
ceramic pieces should be added to even out boiling (if magnetic stirrer is
unavailable). Reaction mixture is refluxed on a sand-bath for 4 hours.
When reaction is done, solution above MnO2 must be colourless. If not, discolouring
may be achieved by addition of 1 ml alcohol or 0,5 g oxalic acid, while heating (this
will quickly reduce excess of KMnO4).
Filter hot solution (through coffee filter) and wash solid with some hot water.
Evaporate filtrate in a beaker to 50-100 ml and filter from newly formed MnO2.
Wash solid with 5 ml hot water and cool combined filtrate to room temperature.
Acidify filtrate with concentrated HCl, until it becomes acidic on pH-paper. This
will precipitate benzoic acid. Filter and wash it with small amount of cold water, dry.
Yield is 5 g (75% from theoretical)
Benzoic acid appears as white crystals with mp of 122oC. In 100 ml of water, 0,27 g
are soluble at 18oC and 5,9 g at 100oC. Also, benzoic acid has good solubility in
acetone.
References
1. S.S.Gitis, A.I.Glaz, A.V.Ivanov, Practical organic chemistry, 1991.
[Edited on 5-10-2008 by sonogashira]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
There is a thread on the oxidation of xylenes to benzen dicarboxylic acids using neutral KMnO4, the conditions described there apply to toluene also.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Can someone help me with finding solubility data for urea in acetone please?
Edit: Thanks solo that's perfect!
[Edited on 7-10-2008 by sonogashira]
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
REFERENCE INFORMATION
A Study of the Equilibrium and Kinetics of Urea Binding by a Biomimetic Dinickel(II) Complex
Sergey V. Kryatov, Elena V. Rybak-Akimova, Franc Meyer, Hans Pritzkow
European Journal of Inorganic Chemistry Volume 2003, Issue 8 , Pages1581 - 1590. 2002
[Excerpt
..............Urea Solubility: The solubility of urea in acetonitrile and acetone. in the range of 0 30 °C was determined by gravimetric.......
Attachment: A Study of the Equilibrium and Kinetics of Urea Binding by a Biomimetic Dinickel(II) Complex.pdf (191kB)
This file has been downloaded 1040 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
My question is: Is CO2 a suitable inert gas for catalytic transfer hydrogenation? With fear i saw dry (unreduced) Pd/C catalyst ignite when letting it
fall into a KCOOH/HCOOH mix.
Next question is: I assume that CO2 is not a suitable inert gas for reactions involving LAH, am i right?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
CO2 is a acid! It will react with LAH...
For the CTh, i would think it will be ok. It must have some kind of influence of the reaction, forming a equilibrium between CO2 (g), CO2 (aq) and
HCO3- , but I don't know if that would really have an impact on the final yield and reaction time.
Why not use N2? It's cheap and very usefull for LAH (although Argon is better, but more expensive).. I really wouldn't feel confortable doing a LAh
reduction without dry, inert atmospher. It's also very practicla to transfert solution via canulas etc
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Random question but here we go!
You know when you dissove NO2 in nitric acid it goes bluish green... How can i remove that colour, in other words, make the NO2 react with the water
to concentrate the HNO3...
I have heard of sweeping it with air, how does that work?
cheers!
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Concentrated nitric acid is 69-71% (about 70-71% if it releases faint white fumes when you blow over the bottle). 100% concentration is obtained when
you distil KNO3 from H2SO4 etc this is white fuming nitric acid and isn't very stable. When you dissolve NO2 in anything over 70% you technically have
red fuming nitric acid (technically RFNA has to have a certain % of NO2 dissolved) RFNA is much more stable that WFNA. To remove the NO2 to get WFNA
from RFNA you warm it up and blow a current of warm dry air through it. I have a feeling that addition of urea decomposes nitrous acid which is formed
and which makes the WFNA unstable for storage. 2NO2 + H2O => HNO3 + HNO2
Generally all you will ever need is 69-71% "concentrated" acid which is constant boiling and the usual concentrated acid from suppliers! Only make the
rediculously strong stuff RFNA and WFNA as and when needed by distilling form H2SO4 etc (plenty of literature on these things)
Edit: NO2 is really interesting molecule since it forms N2O4 (colourless) when cooled or put under pressure (can be done in a nice glass syninge) of
if expanded and heated it turns almost black when all of the molecules are in the NO2 state! really fun! The NO2 brown colour is actually due to an
equilibrium between the two molecules. Liquid NO2 :. is actually N2O4 and is great fun to add to strong reducing agents...(it's reacted with hydrazine
derivatives in rocket fuel, can be done more afely with aniline and NO2 gas!)
[Edited on 7-10-2008 by panziandi]
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
2 questions:
Why does biuret and other peptides form red complexes with copper? Ive never seen other colors than blue/green/purple with copper in the 2+ oxidation
state.
What solvent/temperature/reaction conditions are needed in the HgSO4-catalysed hydrolysis of acetylene yielding acetaldehyde?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Jor
What solvent/temperature/reaction conditions are needed in the HgSO4-catalysed hydrolysis of acetylene yielding acetaldehyde? |
It's not hydrolysis, which implies the splitting apart of the reacting molecule, but addition
for lots of old patents on the process
http://tinyurl.com/46625e
more recent data from Hydrocarbon Chemistry by George A. Olah, Árpád Molnár - 2003
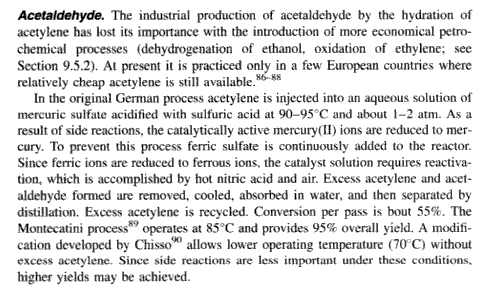
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
water in the manometer-woops
I was calibrating my aspirator pump. I left the room and a sudden, I don't know what exactly, event caused my trap and my lines to load up with
water. My manometer got water in it too. The question is, after I dry out the tubing, do I have to do anything to the mercury? What's the best way to
dry the tube? Acetone?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
| Quote: | Originally posted by chemrox
I was calibrating my aspirator pump. I left the room and a sudden, I don't know what exactly, event caused my trap and my lines to load up with
water. My manometer got water in it too. The question is, after I dry out the tubing, do I have to do anything to the mercury? What's the best way to
dry the tube? Acetone? |
You can only calibrate something that is used to measure, such as your manometer, but thats just being pedantic, lol. If your water is crap then you
may wash your mercury with dilute nitric (1M) in a sep funnel to remove annoying salts but really its not necessary, your main source of error in this
situation is the coarseness of the u-tube's internal diameter and this will overwhelm any error caused by anions dissolved in the mercury. Dry your
tube in the oven i would think, if its contaminated with mercury wash it with dilute nitric also.
I have a question also, what does 'fused' mean in relation to ionic compounds, is it simply chunks formed from the melt? What generally are the
properties of a fused cmp that make it different to a non fused one?
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Mercuric salts and acetylene:
http://dx.doi.org/10.1021/ja01442a010
Fused generally means melted together.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Fusion of ionic compounds generally means fairly high temperatures, driving off all volatile substances such as water. Fused ZnCl2 would be an
example, sodium acetate another. There's an implication that the compound itself is pretty stable to fusion.
Sometimes 'fused' relates to a method of preparation and level of purity, technical sodium sulfide as fused flakes. There's no standard on that, you
need to know the terminology for each compound.
|
|
|
epeters
Harmless

Posts: 3
Registered: 9-10-2008
Location: LA
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Jor
2 questions:
Why does biuret and other peptides form red complexes with copper? Ive never seen other colors than blue/green/purple with copper in the 2+ oxidation
state.
? |
1. are you sure its 2+ copper?
2. other considerations for color:
the thickness of the surface material (if its a thin film) will cause only certain wavelengths to be reflected. this is the case in most oxides (put
a penny in the oven and it will turn red at some point, blue, yellow, green, at others)
this is only a guess, but typically when metals are bound to peptides, they change the conformation of the peptide and the overall energy of the
bonds.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
I don't think it's a thin film, I think it's a soluble complex. But maybe you're right.
I was looking at my bottle of iodoform, and was thinking, comon there has to be something you can do with besides testing for acetaldehyde or methyl
ketones, and decomposing it to give nice iodine and carbon wich destroys the test tube.
I had a try at diiodomethane. Take some of the beautiful glinstering crystals (didn't filter when making it, but extracted with DCM and evaporated,
when i filter I get powder), added a large crystal of KI, a mL of water and next about 3mL of 85% phosphoric acid. My idea to generate HI in site, and
reacting that with the CHI3, to form CH2I2 and I2. No success, just a golden/orange beautiful liquid, with those glinstering crystals in it.
Is it possible to make diiodomethane via iodoform, and how, not using HI , or even I2/Red P (wich I have both though).
Are their any other uses for iodoform?
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Is a variac the best way to vary the temperature in a heating element?
Does anyone know how commercial vacuum lines/systems prevent the entire manifold from losing vacuum if one accidentally/intentionally opens the system
to the atmosphere?
Also is there a quick no-brainer, non-destructive test to differentiate stainless from platinum. The item is a fine meshed electrode or sieve, i'm
almost certain its not platinum but the mesh has been soldered onto the frame and very little distortion or discoloration exists in the mesh
prompting my speculation it may not be stainless, also someone has spent a lot of time on making it....
It doesn't rust up after dipping in 30% hcl and allowing it to air dry. I don't want to heat it to melting point as the solder will melt off and i
can't solder for shit so i would have to get it repaired...
[Edited on 9-10-2008 by Panache]
[Edited on 9-10-2008 by Panache]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Jor...
Is it possible to make diiodomethane via iodoform, and how, not using HI , or even I2/Red P (wich I have both though).
|
Start with http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1... which has several other methods mentioned in the footnotes.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
| Quote: | Originally posted by Jor
Is it possible to make diiodomethane via iodoform |
Like the acetaldehyde question, this is answered in the Systematic Organic Chemistry .djvu that no one reads. It is also in Adams' Organic Chemical
Reagents, and some obscure thing known as Organic Syntheses. But why read when people will just spoonfeed you, eh?
[Edited on 9-10-2008 by S.C. Wack]
|
|
|
raiden
Harmless

Posts: 38
Registered: 4-2-2008
Member Is Offline
Mood: Curious
|
|
Does anyone know what happened to Frogfot's website?
Thankfully it has been retained on archive.org, but I have not seen the man post lately and he expressed no intention of shutting it down.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by S.C. Wack
| Quote: | Originally posted by Jor
Is it possible to make diiodomethane via iodoform |
Like the acetaldehyde question, this is answered in the Systematic Organic Chemistry .djvu that no one reads. It is also in Adams' Organic Chemical
Reagents, and some obscure thing known as Organic Syntheses. But why read when people will just spoonfeed you, eh?
[Edited on 9-10-2008 by S.C. Wack] |
Im sorry , but I can't open DJVU-files. Have to download the codec sometime.
I didn't know orgsyn upto now. Very good site!
Thank you , I will find it now.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I've just checke dthe French ref in the OrgSYn article on CH2I2, and it looks to me as if it's one of the most convinient ways of preparing methylene
iodide, apart from the DCM/KI (the J. Chem. Soc. ref in the OrgSyn note seems wrong, leading to "Resolution of dl-trans-cyclopentane-1 :
3-dicarboxylic acid" . i'm surprised there isn't any more recent refs on the subject, I guess everyone likes playing with arsenious oxide (not me).
Partial Translation of Ann. Chim. Phys., , 53 (3), 313 (1858)
| Quote: |
Memoire on methylene iodide
by M. Alexandre BOUTLEROW
Professor of Chemistry at the University of Kasan
Considering that sodium ethoxide has a similar constitutuion to sodium hydroxide, I thought it would be interesting to compare the action of iodine on
these two compounds, so I studied the action of this simple compound on the ethoxide.
When powdered iodine is added in portions to cristallized sodium ethoxide, a vigorous reaction immediatly occurs, followed by heat evolution: the mass
liquifies and turns brown; but after a while, as soon as all the iodien has reacted, this coloration disappears. By continuing the addition of iodine,
we fianlly obtain a thick and yellow magma.
For the complete decomposition of the ethoxide to occur, and the brown color indicating excess I2 to persists, more than one equivalent of iodine for
each equivalent of ethoxide are required. When the mixture is distilled on a water bath, some alcohol is collected, containing a heavy oily compound
which can be seperated by addition of water. When diluted with water, the distillation residu dissolves nearly entirely, leaving some iodoform. The
aqueuse solution contains some sodium iodide and sodium formate, but no iodate. By evaporating this solution and distilling the inorganic residu with
tartaric acid, some formic acid can be detected in the distillate, using a silver salt. At the same time the alcohol seems to be regenerated in this
recation, that we can surely express by the following equation:
8 C4H5O2Na + 16 I = C2HNaO4 + 6C4H5O2H + 3 C2HI3 + 7NaI (?? apparently they hadn't worked out the structures completely yet!)
By using one equivalent of iodine for each equivalent of ethoxide, and by gradually distilling to near-dryness, a quite large amount of oil is
obtained in the distillate, compared to the amount of iodoform that stays in the residu; on the other hand, the latter is found in exces if the yellow
magma is directly diluted with water without been submitted to distillation. These observations seem to indicate that the oily compound is only a
by-product from the action of sodium ethoxide on iodoform. I had to study this action.
On solid iodoform container in a large container, is added a mildly-concentrated solution of sodium ethoxide obtained by dissolution of sodium metal
in a rather large volume of absolute ethanol. Brief warming in a hot water bath is needed to get the reaction started. The mixture quickly heats up to
a vigorous reflux, with no gas evolution. Once the reaction is finished and the liquor is no longer alkaline, a new portion of ethoxide is added and
the mixture is heated; these operations are repeated until 3 equivalents of ethoxide (based on the amount of sodium dissolved) are added. At this
moment, the liquid is slightly basic, but remains so. It is heated for another few minutes, then diluted with water. A yellowish, milky solution is
obtained, and after a moment a brown oily substance crashes out of solution. When the reaction is well performed, the oil contains little impurities;
but is heating is excessive, or that a too alrge excess of ethoxide has been employed, the oily substance is contaminated with a brown decomposition
product, pulveresent and insoluble in alcohol.
On the opposit, unreacetd iodoform remains if heating was insufficient or that too little ethoxide was added.
Exces alcali seems to prevent deposition of the product; the addition of a few drops of acid easily induces deposition. After 24h, all the oil has
crashed out, and the aqueous solution become colorless. The oil is decanetd, washed and readily steam-distilled.
The compound thus obtained is identical to the oil obtained by the action of iodine on sodium ethoxide, as confirmed by elemental analysis. When
distilled, it boils around 181°C, but is partially decomposed, loosing iodine. The distilled product is always colored, and during the distillation
the head temperature increases as the residu darkens more and more.
Afetr having been distilled in steam and dried over fused calcium chloride, the compound thus obtained presents the following caracteristics: it is a
yellowish oil, very refractive, possesing the surprising property of hardly wettening glass; it's smell is analogous to that of chloroform, and
reminds taht of ethyl iodide, it taste is very sweet (!!). It the denser of all organic substances, it's density at 5°C equals 3.342; at the
temperatur eof +2°C, it solidifies in large shiny shards, that only melt at +5°C. the solidification, once started, continues even at 3°C. During
cristillazation, there is a very significant reduction of volume. It's dilatation coefficent is very high [...]. This compound is not atatcked by
concentrated KOH or by hot mildly-concentrated nitric acid.
The analysis leads to te following structure:
C2H2I2,
which represents the methylene iodide [...].
[...]
A few months ago, M Brüning published in the Annalen der Chemie und Pharmacie, the results his work in M Strecker's laboratory, where he describs a
product obtained by the action of ethanolic potash on iodoform. This product posses all the properties of methylene iodide: same density (3.345), same
boiling point (181-182°C), same % of carbon and hydrogen obtained by titration. But the quantity of iodine found by M. Brüning is too small, which
lead him to suggest the structure
C2H2I2O,
very unlikely for a condensation corresponding to 4 volumes of steam (?). Not having enough material at my disposition, I could not measure the vapor
density which is very simialr for both structures. [...]
Wanting to investigate the variosu products formed at the same time as methylene iodide, I evaporated the aqueous solution decanted from the oil.
After having checked it contained, apart from sodium iodide, salts of volatil organic acids, I distilled the residu with excess tartaric acid. The
distille dsolution was strongly acidic, and droplets of oil where noticed on the surface. Neutralized with Ba(OH)2, it gave afetr evaporation a
inorganic residu that I failed to cristallize. The carbon, hydrogena nd barium contents seem to indicate presence of volatils fattya cids of teh
series CnHnO4.[...]
These results indicate beyond doubt the presence of fatty acids of considerable molecular weight, being at least valerianic acid. On the other side,
we witnessed the presence of formic acid in the distillates. This synthetic formation of molecules having up to 10 or even 12 carbons by the reaction
of two compounds which contain 2 and 4 carbons, where the temperature does not exceed 100°C, seemed to me as being worth mentionning.
|
[The rest of the article then details the decomposition of methylene iodide, it's reaction with Ag(OAc)2 to give (AcO)2CH2 and the authors attempst to
isolate the diol HOCH2OH.... ]
[Edited on 10-10-2008 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
The Rhodium page that has the JCS article's procedure says page 1408 instead of 1400, though which page exactly this is on is unclear without looking
at pdf. The page is at Erowid as usual and the preparation has been attempted here before in a thread with the title of Methylene Iodide.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Wow! That is a very dense liquid. I have never seen a liquid wich is denser than this, except for mercury. Pretty cool.
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
I'm looking for a West or Liebig condenser which are pretty much the same right?
I need it for a 24/40 basic distillation set up what is a good standard length 200mm, 300mm, 400mm or 600mm?
I realize the longer ones will be more efficient but which one is most practical?
|
|
|
| Pages:
1
..
3
4
5
6
7
..
28 |