jmister28
Harmless

Posts: 22
Registered: 12-12-2018
Member Is Offline
|
|
how could I synthesize this molecule
Hi, I have an attached image below of a molecule that I have been trying to synthesize. I'm having trouble finding the best precursors and catalysts.
Any Ideas?
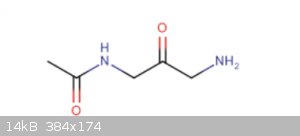
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
I hope it is nothing illegal:
Acetone ---> Dichloroacetone ---> Diaminoacetone ---> Diaminoacetonemonoacetate
---> Aminoacetoneacetamide or whatever it is called.
Not sure how acetone chlorinates though, perhaps you will just get 1,1-dichloroacetone instead of the desired 1,3-dichloroacetone. Be careful with
chloroacetones.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
can't you just do a ketonisation reaction between glycine and n-acetyl glycine ?
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Perhaps under vacuum but i very much doubt it would not decompose into polymerized ketone and imine crap.
|
|
|
jmister28
Harmless

Posts: 22
Registered: 12-12-2018
Member Is Offline
|
|
what reagents would you use for:
dichloroacetone --(???)--> diaminoacetone --(???)--> diaminoacetonemonoacetate --(???)--> Aminoacetoneacetamide
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Besides the chloroacetone (which i suggest you find an alternative to such as iodoacetone) it is step by step:
Step 2: (Nucleophilic substitution) Aqueous ammonia in excess
Step 3: (Acid-Base neutralization) Acetic acid of any concentration
Step 4: (Dehydration) Nothing, just heat carefully. If it decomposes it may need catalyst and/or vacuum.
Really though it may be pointless because Haloacetones are nasty, i would not try it without a good fumehood and judging from your knowledge of these
reactions; more experience.
https://en.wikipedia.org/wiki/Bis(chloromethyl)_ketone
http://www.sciencemadness.org/talk/viewthread.php?tid=18579
Maybe if you could protect a carboxyl group on citric acid you could get to diaminoacetone that way via Hofmann degredation but i can not think of any
way to just protect the middle carboxylic acid.
[Edited on 20-12-2018 by Σldritch]
|
|
|
jmister28
Harmless

Posts: 22
Registered: 12-12-2018
Member Is Offline
|
|
so using these I would end up with Aminoacetone acetamide?
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
1,3-diaminoacetone is prepared from acetonedicarboxylic acid via 1,3-diisonitrosoacetone. It is described in Preparation of organic intermediates by
Shirley. It is easily downloaded from t'internet. Good luck with the acetonedicarboxylic acid! I have a lot of experience with it, most unsuccessful.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
I would start from glycine --> BOC protection of glycine amino group --> acid chloride from glycine with cyanuric chloride and TEA (see here: http://www.sciencemadness.org/talk/viewthread.php?tid=80658&...) or TCCA and triphenylphosphine (see here: http://www.sciencemadness.org/talk/viewthread.php?tid=80658&...) --> reaction with acetamide --> BOC deprotection of the amino group and you
would get your molecule.
[Edited on 26-12-2018 by Chemi Pharma]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
firstly, you can't acylate amides that way -https://pubs.acs.org/doi/abs/10.1021/ja01535a032
and secondly,even if it worked,you still would be missing the carbon in the middle.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
@Cureus, You're right. I can't believe I didn't see the carbon in the middle. Forget all what I've said about the synthesis. May be other way would be
start with 1,3 dichloroacetone (sold by Merck/Sigma Aldrich), make a delepine reaction with HMTA to obtain the ketone diamine and reacts the last with
a stoichometric amount of acetyl chloride to N-acylate only one aminogroup, giving the OP's desired product.
On the other hand, I think is possible to do an N-acylation on the amide with an acid chloride, the same way the literature show examples of the
N-alkylation and N-arylation with alkyl chlorides and aryl halides cause the mechanism is the same. See: https://www.organic-chemistry.org/synthesis/C1N/amides2.shtm... Also, this article tell about the diacylation of amides with acyl chlorides: https://pubs.acs.org/doi/abs/10.1021/ja01156a115. Could you explain to me why you think the N-acylation won't work in this case?
[Edited on 26-12-2018 by Chemi Pharma]
|
|
|
Jon snow
Harmless

Posts: 11
Registered: 6-1-2016
Member Is Offline
Mood: No Mood
|
|
I don't frequently browse the forum so please correct me if I'm wrong but I don't think it's generally a good idea spoon-feeding information regarding
drug manufacturing and the production of drug precursors like the molecule op mentioned
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Jon snow  | | I don't frequently browse the forum so please correct me if I'm wrong but I don't think it's generally a good idea spoon-feeding information regarding
drug manufacturing and the production of drug precursors like the molecule op mentioned |
What is this a precursor for?
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Jon snow
Harmless

Posts: 11
Registered: 6-1-2016
Member Is Offline
Mood: No Mood
|
|
It's a precursor to a variety of Imidazobenzodiazepines like climazolam and midazolam if I remember correctly, the other precursors being the
corresponding 2-aminobenzophenones
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I would hardly consider this a "precursor". Also, after looking at the structures of those compounds you mentioned, while i see some similarity, I
dont see a direct conversion of this to those medications.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Jon snow
Harmless

Posts: 11
Registered: 6-1-2016
Member Is Offline
Mood: No Mood
|
|
I'm not an expert on the chemistry behind the synthesis of benzodiazepines but I'll provide a link to a patent regarding the synthesis of midazolam
from the compound in discussion, it looks pretty direct to me but I'm not sure because it looks like poorly translated and English isn't my first
language
https://patents.google.com/patent/WO2016146049A1/en
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I see what you're saying. I knew that I would eat my words.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|