amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
Substituted quinazolines
In order to substitute the chlorine with different anilines the author used acidulated water or benzene
I don't have the paper so I don't know the details but from my experience I know that this ester is very reactive and can form amide with amines
easily
I am just wondering .........does this reaction proceeds at rt or needs reflux (step 2) and how to avoid reaction between ester and amine if reflux
was used
[Edited on 12-11-2008 by amrhamed2]
amr h mahmoud
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
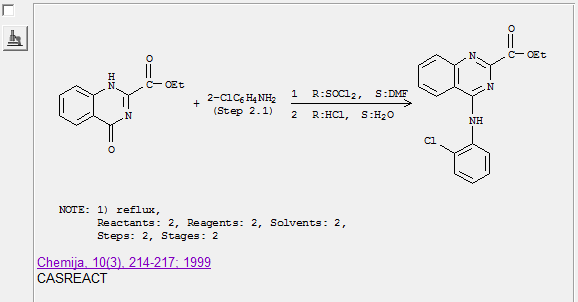
Here is the abstract of the paper
Synthesis and antiinflammatory activity of ethyl 4-(anilino substituted)-2-quinazolinecarboxylates. Mekuskiene, Giedrute; Udrenaite, Emilija;
Gaidelis, Povilas; Vainilavicius, Povilas. Faculty of Chemistry, Vilnius University, Vilnius, Lithuania. Chemija (1999), 10(3), 214-217.
Publisher: Academia, CODEN: CHMJES ISSN: 0235-7216. Journal written in English. CAN 133:362749 AN 2000:675531 CAPLUS
Abstract
Treatment of Et 4-oxo-3,4-dihydro-2-quinazolinecarboxylate with thionyl chloride resulted in Et 4-chloro-3,4-dihydro-2-quinazolinecarboxylate
formation. The latter reacted with arom. amines in acidified water or benzene to form Et 4-(anilino substituted)-2-quinazolinecarboxylate possessing
antiinflammatory activity.
amr h mahmoud
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Aminolysis of desactivated esters with aromatic amines requires very hot temperatures and oftene xcessive reaction times.
When adding SOCl2 to the substarte, youa re forming a chloro-iminium species, which is very reactive and will attack the amine long before the ester.
So no worry on that side IMHO.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|