Intergalactic_Captain
Hazard to Others
  
Posts: 227
Registered: 4-9-2004
Location: somewhere where i don\'t know where i am
Member Is Offline
Mood: frabjous
|
|
JWH-018 - Proposed Synthesis
JWH-018, a synthetic alkyl-amino-indole cannabinoid, has recently been found to be the main psychoactive ingredient in many of the newest generation
of "legal highs." Here's the link to the report issued by THC-Pharm, the German firm that was contracted to run the analysis.
http://usualredant.de/drogen/download/analyse-thc-pharm-spic...
Having tried several related products, including one in the report, I can attest that the compound is indeed active and eerily similar to the natural
drug it is trying to mimic. Now that I know what I ingested, I've been working on a proposed synthesis.
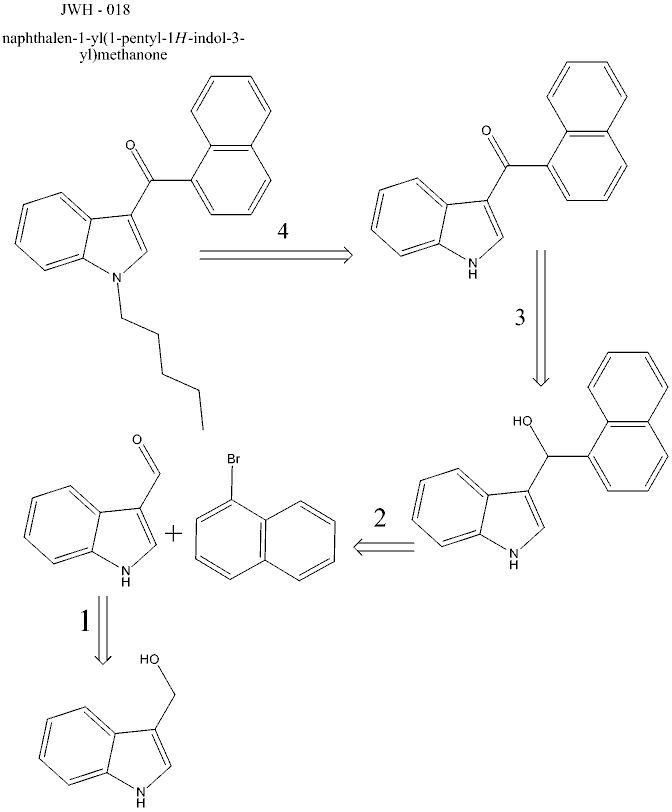
1 - Oxidation of Indole-3-carbinol to 3-formyl-indole. I'm not particularly farmiliar with indole chemistry, but I'd imagine that being aromatic I'd
imagine that it's analogous to benzyl alcohol for this reaction. PCC should work, but I'd rather avoid pyridine. I've found a couple ref's
suggesting the ability to stop a Jones reagent from taking it all the way to the carboxylic acid, though;
| Quote: |
JOC 40(11), 1664-1665, 1975
Selective Oxidation of Allylic Alcohols
with Chromic Acid
Jones Oxidation of Cinnamyl Alcohol. A solution of 500 mg
(3.72 mmol) of cinnamyl alcohol and 10 ml of reagent-grade acetone
was placed in a 50-ml round-bottom flask under nitrogen and
cooled to 0' (ice-water bath). To the magnetically stirred solution
was added dropwise a solution consisting of 2 ml of 8 N Jones reagent
and 18 ml of reagent acetone. The Jones solution was added
over a period of ca. 20 min until an orange tint persisted in the
reaction mixture. Isopropyl alcohol was then added dropwise to
destroy excess Jones reagent, as indicated by the reappearance of a
deep green color. The reaction mixture was then extracted twice
with ether, and the combined ether extracts were washed (water,
sodium bicarbonate, and brine), dried over anhydrous magnesium
sulfate, and concentrated. Evaporative distillation (0.1 mm, 100')
yielded 420 mg (2.96 mmol, 84%) of a cinnamon-smelling, pale yellow
oil (>92% pure by GLC) identified as cinnamaldehyde by comparison
of the ir and NMR spectra with literature spectra.
Jones Oxidation of Benzyl Alcohol. A solutipn of 500 mg (4.63
mmol) of benzyl alcohol and 10 ml of reagent-grade acetone was
placed in a 50-ml round-bottom flask and cooled to 0' (ice-water
bath). Oxidation in the same manner gave material which upon
evaporative distillation (water aspirator pressure, looo) yielded
380 mg (3.52 mmol, 76%) of a clear oil (>99% pure by GLC) identified
by ir and NMR as benzaldehyde. |
And, the "Silica Gel Supported Jones Reagent," made by mixing silica gel with the Jones reagent. Supposedly capable of converting benzyl alcohol to
benzaldehyde in 85% yield.
http://www.erowid.org/archive/rhodium/chemistry/alcohol2alde...
Step 2 - Grignard reaction between 3-formyl-indole and 1-bromonaphthalene. Again, not too farmiliar with indole chemistry, but the N-proton shouldn't
screw with the grignard reagent, would it?
Step 3 - Oxidation of the obtained alcohol to the ketone. Plan is to use Jones reagent.
Step 4 - N-Alkylation to JWH-018. Plan to use same conditions used by J.W. Huffman in original published procedure....I can't seem to find the
article at the moment, but IIRC it was n-bromopentane, KOH, and the naphthoyl-indole in DMSO at 80C. I'll replace that with the published procedure
when I find it.
This seems simple enough, perhaps even a wal-mart synthesis. Indole-3-carbinol is sold as an anti-cancer supplement for ~$.50-2.00 US / gram by
various online health stores, though I've yet to find a local source. Potassium Dichromate has been on my shopping list for some time, and though not
necessarily something I can grab at the store, can be had rather cheaply from ebay. The CrO3 required for the Jones reagent can be had from H2SO4 +
the dichromate, though I wonder if it would need to be isolated or if the mixture could be used directly?
n-bromopentane would be prepared from n-pentanol and NaBr/H2SO4.
The only thing I haven't worked out yet is the 1-bromonaphthalene... I've found a few articles indicating that it can be made from naphthalene in
moderate yeild via Na/KBr and oxone, will post if anyone's interested. Considering I've got a gallon of 27% peroxide and pool season's over (no more
oxone locally), anyone have a procedure utilising it?
EDIT - Had to resize the attached pic.
[Edited on 12-21-08 by Intergalactic_Captain]
If you see me running, try to keep up.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
That's not the way how one would make a 3-acylindole.
A grignard would simply deprotonate the indole to the indolyl cation. If it would react with the carbonyl before that I cannot tell, but acid-base
reactions like the deprotonation sure are one hell of a lot faster.
Remember Anthony-Speeter tryptamine synthesis, first step?
Combine 1-naphthoyl chloride and indole in ether. If that doesn't work, use a FC catalyst, preferably one that doesn't release HCl to chew up the
indole.
1- Naphthoyl chloride is made from a-bromonaphthalene via grignard + CO2, then thionyl chloride on the naphthoic acid.
a-bromonaphthalene see Orgsyn.
http://www.orgsyn.org/orgsyn/pdfs/CV1P0121.pdf
CAUTION: The drawing of the compound is WRONG here. Also, the nomenclature is contradictory. a-bromonahthalene is 1-bromonaphthalene, not 2-.
This reaction (naphthalene + bromine in boiling CCl4) yields the desired a-bromonaphthalene, I have looked this up.
If you doubt it, just compare the stated boiling point of the product with literature values for a- and b- bromonaphthalene.
As often, CCl4 can likely be substituted by CHCl3 or DCM.
Alkylation is probably trickier... phase-transfer catalysis with pentyl bromide and aqueous base is perhaps possible.
Or in DMSO.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The synthesis of this compound is already published in the scientific literature (and it is a two step synthesis that makes much more sense to what is
proposed above).
I will not tolerate any threads that make publicity to a (cynically) so called "research chemical" drug. The abuse of law for making profit is
contradictory to the values this forum promotes (amateur science). I rather see discussion about the synthesis of illegal compounds than this! Any
thread promoting "RC drugs" will be closed. If anybody is too stupid to realize the reasons for this... well, get another hobby!
|
|
|
|