Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
Thermal rearrangement of imine to amine (with microwave?)
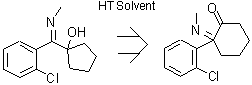
One reaction im studying requires a thermal rearrangement to convert it from an imine to an amine. This requires the use of a high temperature solvent
like decalin or ethyl benzoate. I'm wondering whether this would be possible through microwave methods instead? Does anyone have any reference to this
kind of process if it exists?
I've attached the molecule in question
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
It's still an imine and carbon can only have up to four sticks. Perhaps a reference would be useful.
Best,
O3
[Edited on 22-12-2008 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
well spotted! I've redrawn it so it appears how it should.. as the amine.
thanks in advance
Rettached. apologies for that. I wish preview page previewed with the image
[Edited on 23-12-2008 by Aubrey]
[Edited on 23-12-2008 by Aubrey]
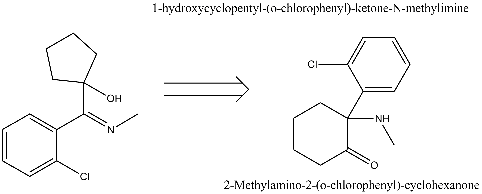
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Aubrey
One reaction im studying requires a thermal rearrangement to convert it from an imine to an amine. This requires the use of a high temperature solvent
like decalin or ethyl benzoate. I'm wondering whether this would be possible through microwave methods instead? Does anyone have any reference to this
kind of process if it exists? |
Are you perhaps saying that you are unable to obtain a high boiling solvent, but at the same time you have access to a microwave reactor?
Anyway, yes a vessel for a microwave reactor is a closed vessel (autoclave) which generally can withstand up to 20 bars or more (depending on the
reactor type). For up to 200°C you can use almost any solvent that has a bp > 80°C at atmospheric pressure and you will not cross over the
pressure limit.
PS1: Please provide references when opening new threads! A simple googling gave a list of references for this rearrangement already in the first hit
(http://www.erowid.org/archive/rhodium/chemistry/pcp/ketamine...). Given the end compound, ketamine, is a widely used veterinary anaesthetic I'm
sure there are plenty of synthesis examples in the literature and patents, so why don't you read about it? You obviously know almost nothing about
organic chemistry or else you would not draw a five valent carbon - therefore reading some literature would do you good.
PS2: Please edit the picture above. It is too wide and it distorts the forum frameset for those of us who use the most common screen sizes.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
thanks for your response nicodem, i have indeed much of the literature on the substance and have looked though what would be most relevant topics in
Lindstroms' 'Microwave assisted organic synthesis'. From what i can ascertain most microwave methods use the freq of conventional microwaves.
There is a section on reduction of imine and a section on the Beckmann rearrangement, but since the literature on erowid doesnt state what kind of
thermal rearrangement is occurring, I was hoping someone more skilled than myself might recognise it.
I will have the high temperature solvent at some point, whether i make or puchase, but the pospect of peforming one step in the reaction in a matter
of a few minutes rather han several hours sounds too good to miss, it would be cleaner , and cheaper on both electicity cost and solvents, and would
mean i wouldnt need to worry so much about knocking over my towering alihn condenser.  But as always i am reading, learning. But as always i am reading, learning.
[Edited on 23-12-2008 by Aubrey]
|
|
|
harrydrez
Harmless

Posts: 26
Registered: 28-11-2008
Location: usa
Member Is Offline
Mood: content
|
|
Maybe I'm missing something here, but are you looking to use a home microwave as a lab microwave device? I have one in my lab (at work), it's a
godsend but it's specifically designed for reactions (we use ours for extractions mostly).
|
|
|
Aubrey
Harmless

Posts: 37
Registered: 16-11-2008
Member Is Offline
Mood: No Mood
|
|
Here's an excerpt from the aforementioned book. I'll see if i can dig up the paper they are referring to which may give moe detail. Harrydrez, yes im
looking at using a home microwave if at all possible. it seems like no solvent is required in rearrangements although sample size does make a
difference. id be interested to know how you got on with your reactions, but i guess theres thousands of papers on the subject i should read first

6.3.15. Ketone–ketone rearrangements using polymer-supported AlCl3
A recent publication from an Indian group has described the synthesis of a small selection
of 3-(4-alkoxyphenyl)-3-methylbutan-2-ones, which are useful starting materials
in the synthesis of a wide range of natural products. A polymer-supported aluminium
chloride species159 was used to catalyse the rearrangement of ketone 15, followed by
O-alkylation of the trapped intermediate with simple alkyl halides (bromo and chloro)
as well as dimethyl and diethyl sulphate (Scheme 6.21)160. Both transformations were
found to occur with improved efficiency, when the heating was conducted using microwave
irradiation. The authors claim this procedure is the simplest and most economical
methodology used to synthesise these intermediates to date.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I'm afraid you are completely confused with the regard of what is a microwave reactor and what is the point of heating the reaction with microwaves
instead of conventional (convectional) heating. Microwave reactors and kitchen microwave ovens are two different things. You can not heat closed
reaction vessels in a kitchen microwave oven as that would not just get the temperature out of control, but it would also be calling for a suicide.
All you can do with a kitchen microwave oven are solventless reactions (and even that is possible only if using a large alumina heat sink or else you
just burn your reaction mixture). Using it to heat solutions generally gives no advantage to conventional heating and is just pointless. To be able to
use a closed reaction vessel (glass autoclave) a microwave reactor is needed (very expensive!) since only this can monitor the temperature and
pressure, and regulate the power in accordance to your program.
If you have some reference claiming better yields on this specific reaction when performed in a microwave reactor, then provide it to all to read
instead of posting irrelevant stuff like the above (what the hell that has to do with your problem is beyond me). If there is no such reference, then
what is the point of this thread anyway?
Also, what is all that about your troubles obtaining a high boiling solvent similar to decaline? Don't you have gas stations where you live? Is it
possible that you don't even have one single hardware store in your neighbourhood? I find it impossible to believe.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Texium
|
Thread Moved
19-11-2023 at 12:46 |