tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
Calculating concentration
so if i have sulfuric that has a specific density of 1.84g and its mixed with water that has a specific density of 1g. and i weigh 1 cubic centimeter
of this mixture i should have enough information to find the ratio of the 2 ingrediants. so i weighed a cubic centimeter of this sulfuric acid and
water mix and it turns out that it weighs 1.42g
i could say that 1/2 a cubic centimeter of water weighs .5grams and 1/2 a cubic centimeter of my sulfuric acid weighs .92 grams and when you add them
together you get 1 whole cubic centimeter weighing 1.42g
or i could say that i know that my sulfuric acidweighs 1.84 @ 100% and figure that
x/1.42=100/1.84
the first method determines that the ratio is 50:50
and the second method determines that the ratio is 77:23, which method is correct
in other words whats a could formula for calculating concetration?
[Edited on 2-12-2003 by tom haggen]
|
|
|
unionised
International Hazard
    
Posts: 5103
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The first one is right in this case (but I don't know what units you would use for getting milk that dense).
I'm not sure why I bother to mention this since you are on about milk, but the same arithmetic will not work with sulphuric acid. That's why
there are density tables.
The second formula is wrong because it is based on the asssumption that at zero% milk you will have zero density.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
reply
why wouldn't my arithmetic work with sulfuric acid
and i know to look things up in the density tables but i have a mixture of unknown concentration and i'm trying to use known info to figure it
out,
[Edited on 2-12-2003 by tom haggen]
N/A
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
tom haggen, check this (very last message to date)
http://www.sciencemadness.org/talk/viewthread.php?tid=401
I had this discussion with primopyro once, and I reasoned against it... although noone has answered to it yet.
Nonetheless, I don't think my reasoning is flawed, in accordance also with unionised's post 
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
ratio
ok i think i understand somewhat but i'm still having trouble understanding why arithmetic won't work for sulfuric acid so I guess i will go
look up the density tables, does anyone have any good ideas on what to search for when it comes to the density tables,
P.S. sorry for not starting this post in the chemistry section
[Edited on 4-12-2003 by tom haggen]
N/A
|
|
|
unionised
International Hazard
    
Posts: 5103
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If you add a litre of acid to a litre of water you don't get 2 litres of solution.
I have a couple of books with density tables in, but i can't seem to find one on the net. Odd!
If my scanner weren't playing silly games I would send you a copy.
EDIT
Found one
http://www.basf.de/basf/img/produkte/farbmittel/leder/pdf/pb...
H2SO4 is page 7
[Edited on 3-12-2003 by unionised]
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
reply
unionised i apprectiate what you have already posted, i have learned a lot from it. however, the density chart i am seeking is the one for sulfuric
acid, i'm sure eventually I will find a use for the chart on formic acid you already posted though. If you ever get your scanner to work i would
be intrested to see the information contained in your book.
-Tom
[Edited on 4-12-2003 by tom haggen]
[Edited on 4-12-2003 by tom haggen]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
volume change on mixing
Unless the liquids being mixed form an ideal solution there will be a volume change on mixing. In most practical situations this change will exist to
a greater or lesser degree. That is why there are density vs concentration tables published for most of the important industrial mixtures like
sulfuric acid/water. These can be found in the chemistry and chemical engineering handbooks such as Perry's, Lange's, and CRC.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
reply
ya too bad you have to pay for this information. To be more clear about my earlier post, I'm looking for a search term that might possibly take
me to a data base containing these type of tables. hopefully i can find some free information. I did find one site called chemlab.com but there is a
lot of costfull info there. If i look hard i might find some free data so if anyone else is looking for density tables you might check out chemlab.com
and look around
N/A
|
|
|
unionised
International Hazard
    
Posts: 5103
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Read ALL of the message I posted, including the last line.
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
reply
sorry i had my head up my ass unionised
N/A
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
HNO3 Density vs % in H2O
uh, but the most important one in your very useful pdf is missing: HNO3!!
Despite previously unsuccessful attempts, I finally found it, in fact in the roguesci archives!
Have a look in the nitric acid thread for the actual data! http://www.sciencemadness.org/talk/viewthread.php?tid=401&am... , where I posted it to avoid double posting.
However, I would like to discuss a further aspect to it. I plotted the data, see the image
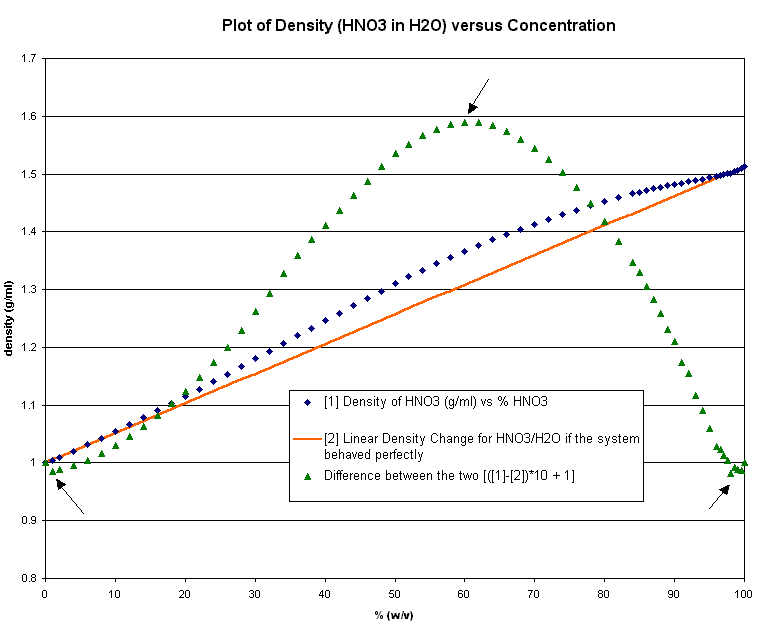
You can see it doesnt even remotely come close to a straight linear process (orange line, as would be expected for perfect fluids), and not even some
kind of log/exponentional function (as the difference between real data and the perfect fluid line indicates, i.e. green).
I was wondering, what is producing the greatest deviation (density increase) at 60% precisely, or density DECREASES close to zero and 100%? They look
fairly unpredictable to me.
I hypothesise this corresponds in part to HNO3*(H2O)n, and in part with the varying degree of ionisation with increasing temperature. I.e. at 100%
ionisation will be H+ and NO3- (if that), while, at 60%, it will be [H3O+] + [NO3-]. This in turn relates to the stoichiometry ratios between water
and HNO3, plus the rates of ionisations... but I struggle to explain the density DECREASES at 2 and 98%!
what do you guys reckon??
Sorry the pic is a litle big, but still just 10 kb 
[Edited on 6-12-2003 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
reply
you guys are awesome, as for the graph if i has to guess i would say the reason for the random density to concentration ratios has to do with quantum
physics
-tom
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
I wouldnt call it random, there is definitely somethign behind it!!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
blip
Hazard to Others
  
Posts: 133
Registered: 16-3-2003
Member Is Offline
Mood: absorbed
|
|
Maybe the density is lower at the extremes because the packing arrangment isn't as efficient, but higher near the middle because it is moreso.
This is just a guess here, though.
|
|
|