| Pages:
1
2 |
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
Alcohol from wheat
The legality-issues aside: How is it possible to make C2H5-OH from wheat (raw delivery, not flour) ?
Behind the question: In EU 1000 kg, directly from the producer, coast only 110 EUR (thats right: 11 cent/kg); with a good conversion-mechanism it
would be good for car-fuel (most modern car-engines of gasoline-type are 100% capable of running on ehtanol, since it's an maybe-upcoming standard
(methanole too)) ...
This is gonna come up anyhow in the future, so why wait for the government to tax it ? Please no ethical counter-argumentation, since the 3rd world
hungers despite world-over-production of food , for political reasons ...)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
By using an obscure process known as brewing.
1. malt the grain
2. roast the malt
3. crush the malt
4. add to sterile water to make the mash
5. take the mash through a controlled temperature sequence to activate the various enzymes the hydrolyse the carbohydrates and to some extent
proteins. you want a β-amylase heavy rest to convert as much of the carbohydrates to simple sugars as possible.
6. boil the mash, omit adding hops
7. separate the grains and precipitate proteins from the liquid
8. cool the wort, aerate with sterile air, add yeast - a high alcohol tolerating strain
9. allow to ferment
10. separate yeast from beer.
11. concentrate alcohol from beer. pervaporation is gaining favour for the first stage, as it reduces energy input. Corn grits can be used to
dehydrate the alcohol-water azeotrope in the vapour phase, the grits are reusable by drying and when degraded too far are added to the mash.
The spent grains and yeast are used as animal feed, heating and cooling steps arranged so as to reduce energy use. While at one time efficiency was of
lower priority, energy input coming from coal or wood, currently keeping the alcohol from consuming too much energy in its production requires
moderate to large scale facilities to maximize energy recovery from what would otherwise be waste heat.
Might want to look at
http://www.aaccnet.org/cerealchemistry/backissues/1995/72_36...
[Edited on 3-2-2009 by not_important]
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
That document gives a experimental yield of 443 l/tonne barley, close to the theoretical yield of 468 l / tonne ...
How would it be with wheat ? Baley is known, I believe, for very easily given the malt, and containing the right enzymes too ...
Also the sorts of barley are extra-selected for the purpose ...
How would I proceed with the variable average grain from a occasional cheap source ?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Automobile engines before the 1970s were capable of running on ethanol-water mixtures (no petrol) with only very minor adjustments to carburator,
Changes introduced in the 1970s make this now extremely difficult, requiring instead the so called gasahol mixtures of petrol and ethanol, with a
great limitation on economy.
In my opinion it is a major blunder to shift food crops to energy use. Better to focus on non-food crops. The subject gets bogged down in politics as
the policies are driven by subsidization of argro-industrial giants and have little to do with reality, and thus being political are outside of the
scope of this forum.
Sic gorgeamus a los subjectatus nunc.
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
Sauron wrote:
| Quote: | | In my opinion it is a major blunder to shift food crops to energy use. Better to focus on non-food crops. The subject gets bogged down in politics as
the policies are driven by subsidization of argro-industrial giants and have little to do with reality, and thus being political are outside of the
scope of this forum. |
Right on, Sauron. The link between science and government is vey tenuous. Same goes for economcs, except pork production. I can remember when the
world treated reality as real instead of some PC claptrap generated by the special interest groups that have hijacked our democracy. But hush, lest I
get pulverized. And I don't want to ruin a perfectly valid thread...
Regards, Der Alte
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@Sauron: Hear, hear! EtOH is lousy fuel. E.g, 10% EtOH added to gasoline reduces the mileage per gallon by ~ 10%. It's political feel good thing
to say they're doing something...at least biodiesel users recycle used oils. And its good for diesel engines. One last comment; to make EtOH from
grain you basically make beer and distill it to an immature whisky. IMHO you're wasting two worthwhile products by burning it in a vehicle ;^)
[Edited on 3-2-2009 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The inescapable fact is that we are using too much fossil fuel and so called alternative energy cannot make a dent in that consumption. The top 20 or
so altfuel technologies if they all come to druition might add up to 20% total consumption. Therefore, comsumption must come down. A lot of things we
took for granted in the 20th century will simply have to go. A great deal of personal transportation will be among the first casualties. Not today not
tomorrow and maybe not in our lifetinmes but certainly this century.
Sic gorgeamus a los subjectatus nunc.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: |
The inescapable fact is that we are using too much fossil fuel and so called alternative energy cannot make a dent in that consumption. The top 20 or
so altfuel technologies if they all come to druition might add up to 20% total consumption. |
Perhaps. But the U.K. might be able to avoid too much harm, in opinion of some:
http://www.withouthotair.com/download.html
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
If here in EU , where gasoline costs 2 EUR/ liter, the C2H5OH can be had for 25 cents per liter, guess what will be despite the hungering africans ...
(and I could go into _that_ too: They live largely on imported grain, paying with credit money ..., stupid globalization) ...
The water-content of the ethanol could be reduced by distilling over Ca(OH)2, from the hardware store, 25 kg for 3.50 EUR ...
Also maybe it could be dried with Na2SO4 (to be recycled) ??
The car engines these days are made for ethanol-gasoline-mixtures, and also for _clean_ ethanol ... ; maybe one needs 30% more volume of this fuel,
but it should work well, once obtained from the grain ...
Now to the wheat: As I understand, the special thing about barley is that it gives a lot of malt-content, so it's the ideal grain for brewing ...
But how would wheat or other grain be proceeded, for efficiency ?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Same way, or with mostly wheat and some barley or rye.
As I already stated, using corn grits or other high complex carbohydrate plant materials to dehydrate the vapour phase azeotrope works well. Ca(OH)2
isn't going to dry it, you need CaO. As neither that nor hydrated salt formation is used to dry ethanol on a commercial scale, this suggests that
they are not practical or efficient enough.
Distillation, both the concentration of the beer and the dehydrating step, are the major energy consumption steps of making fuel alcohol. This is why
pervaporation is increasingly being used, and why various absorptive processes are being pursued for drying the alcohol.
The paper "Cornmeal Adsorber for Dehydrating Ethanol Vapors" is reprinted here:
http://journeytoforever.org/biofuel_library/ethanol_grits.ht...
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Cellulosic ethanol is doable and does not enter the food chain (and there is a $1.01/gal subsidy). Unfortunately, practice is less efficient than
theory, and the technology that works on the bench is not ready for industrial deployment. If you burn crop residues instead of natural gas or fuel
oil (e.g. sugarcane mill) cellulosic ethanol is a win.
That leaves us with oil crops which have similar food-related problems.
The big problem with non-food oil crops:
The things we don't eat tend to be poisonous.
From the US point-of-view (be that as it may), planting acres of poisonous material (ricinus or jatropha) is a bad idea (homeland security and
all-that). Jatropha is getting a push, but it also contains toxalbumin similar to our pal the castor.
That leaves us with algae (and of course, the best ones, such as Caulerpa taxifolia, are toxic). These are difficult to grow in bulk and dewatering is
costly, energy-wise. Still, I'd keep an eye on algae.
For barley and other grains, crush, boil (to swell the starch granules) add a-amylase* (starch is an a-polyglucan, a b-amylase will go for cellulose)
and ferment with turbo yeast. You could even perform the fermentation under vacuum and heat with a high temperature enzyme (such as Tergamyl from
recombinant lichenformis). This allows for rapid saccharification (Arrhenius kinetics apply), removal of toxic ethanol and opens the possibility of
adding more substrate (fed-batch mode). This sort of thing is referred to as simultaneous saccharification and fermentation or SSF. For SSF, your
saccharification rate will be limited by the tempearture that the yeast can withstand (which I don't recall, off-hand). A two step (with high
temperature saccharification, eg. 90°C) with multiple pots might actually give higher yields per unit-time (stagger the batches).
*note that a pure amylase will only get you to maltose (alpha) or cellobiose (beta). A secondary glucoamylase is required to move all of the way to
glucose. This does not really matter because most amylases contain both enzymes (or demonstrate both activities) and the yeast is perfectly happy with
maltose.
Corn grits do look surprisingly good!
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
| Quote: | Originally posted by not_important
By using an obscure process known as brewing.
|
Ha!
To quote myself
'any fermentable sugar can be converted to ethanol using airborn yeasts, correct hydration and temperature conditions and time. Upping the yeast
concentration via a technique know as 'going to the local brew shop, buying the yeast and adding it to the fermentable sugar/water mix' will speed
things up considerably.'
@sauron. Is it as tedious for yourself as it is to me that no matter how obvious the arguments pertaining to consumption reduction, you still hear all
and sundry prattle on about biofuels/renewables blah blah blah as if they were some kind of silver bullet. (apologies for the cliche count in that
last sentence, we can't all be former journalists)
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
I wasn't talking about global politics ... ; as long as 1000 kg wheat coast me only 110 EUR, I'm gonna think of how to convert this. Besides the real
prices of corn would be even much lower ; but the market is regulated in a manner to counter over-production, because much more can be produced than
anyone needs ...
And as long as government & big oil pull the money out of everybodys pocket it's more than all right to think about ethanol from corn ...
Those against it might answer something useful in the fischer-tropsch-synthesis thread I opened maybe 1 year ago: Making fuel out of coal ... (50 Bar
@ 200 Celsius needed, + catalyzer ; maybe some old car-catalyzer could be used ?)
Besides here in Germany there are even people who directly burn wheat in the oven, since it's cheaper than coal ... ; that's how it is. My mother too
taught me that wasting food would be a sin ; but this comes from times of hunger, which for parts of the world most of the time do not exist any more
... ; the others suffer because of politics, not because of wasting ... ; that politics is partly sponsored by big oil ; so it's even good, to bypass
oil-industry and drive the car with corn, indirectly, as long as no direct conversion is invented ...
[Edited on 6-2-2009 by chief]
[Edited on 6-2-2009 by chief]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chief
Those against it might answer something useful in the fischer-tropsch-synthesis thread I opened maybe 1 year ago: Making fuel out of coal ... (50 Bar
@ 200 Celsius needed, + catalyzer ; maybe some old car-catalyzer could be used ?) |
cobalt, iron, or ruthenium; nickel favours methane. Promoters are added, careful control of the crystal structure and size are important. Cobalt is
more active than iron.
Sulfur poisons the catalyst, iron is less sensitive and is cheaper so it often is used with frequent replacement, or used as a first stage to capture
sulfur, help promote water shift, and do some conversion to hydrocarbons, again with frequent replacement.
The water shift would be important if starting with coal or agricultural wastes as it converts some of the water, formed in the reduction of CO, into
CO2 and H2.
As the product is a complex mix of hydrocarbons, considerable post f-t processing must be done starting with fractionating the crude product. The
lighter hydrocarbons are feed back to produce more syngas and/or injected into the reaction area. The heavier hydrocarbons are cracked as is done for
petroleum, taking additional hydrogen or producing much additional coke; the coke can supplement the coal input. Reforming is needed if Diesel fuel
is not the target, as the predominately straight chain alkanes have low octane ratings
The energy input demand is high, 5 to 7 times the mass of coal as mass of hydrocarbon product. The refining process adds to the energy demand.
This energy demand is important, and one reason for the push for electric vehicles. Burning the coal in a modern generation plant allows around half
the energy in coal to be converted to electricity; compared to 1/5 to 1/7 for F-T. Transmission losses to the consumer are under 10%. For trains this
means that a little under half the energy of the coal ends up as motive power, compared to 5% to 8% (after engine losses) for Fischer-Tropsch produce
Diesel fuel. For automobiles the ratio is a bit lower, as the battery charge/discharge process is only 80% (old tech) to 95% (new tech) efficient.
However the powertrain losses come into play, and the final result is still better than 5 times the input energy for an ICE vehicle path than the more
efficient BEv.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
It would be better to convert cellulose to heptanols or octanols; ethanol isn't good fuel. I hope someone here (in SciMad.Org) figures out the
process. Meanwhile, the Fischer-Tropsche process is a model for the kind of process I'm trying to work on. The WWII Germans were desparate for fuel
os 5-10% conversion efficiency was OK. Since then a number of workers have made CO into methane and methanol.. even higher alkanes. The continuiung
issue is the energy demand in the form of pressure and heat. A catalyst that would facilitate conversion of CO2 to alkanes and light aromatics at
modest temperatures and ambient pressure would solve the two biggest problems simultaneously. It is my continuing dream...However, the ultimate issue
is overpopulatation. With a sustainable human population the planet could tolerate gasolene engines, coal fired plants and modicum of 'pollution'. I
don't have a clue on that one; ask the Ayatollah or the Pope.
[Edited on 6-2-2009 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
chief3
Harmless

Posts: 39
Registered: 29-12-2012
Member Is Offline
Mood: No Mood
|
|
Interesting ; how about the drying of Ethanole ... using calcium hydroxide , as was mentioned above ?
==> If this would work well ... the CaOH could be recycled by burning it to some temperatures ...
Also I have read somewhere about drying alcohol with Na2SO4 ... ; the Na2SO4 is cheap ...
Also: What about using plaster (CaSO4) ... ? This is available in any hardware-store ... and reacts with water ... , by taking it into the
crystal-structure ; afterwards it can be recycled by moderate heat, the kitchen-stove might do it well ...
==> One 25kg-bag of plaster costs maybe 5$, and can bind maybe 5-8 kg of water ... ; so if it could bind _all_ of the water from a 90%
Ethanol-Water mix ... the one bag could be useful to purify 50 to 100 kg of Ethanole ... ... ; anyhow the proper handling would have to be figured
out: Constant stirring for maybe 30 minutes might be required, and the plaster might take some amount of Ethanol into its volume ...
==================
I have dug out this thread because of the biofuel-idea ; it seems to me that sugar would be a too expensive source of primary material ... ; various
sorts of corn cost only 200 EUR/metric ton ... : If such could be converted to ethanole ... maybe it would be worth trying ... , since one would have
to produce it significantly chaeper than commercial biofuel to make it worth going for it ...
==> The E85-Biofuel curently in Germany is slightly above 1 EUR/liter ... , so it would be nice if self-made could be had at around 50 cents ...
... ...
Also in Europe, and specially in Germany, there are historically bans of imported sugar, and price-kartels by law for standard sugar ... just to
ensure a high enough price for the local sugar-makers ... ...
What do you tink ?
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Slightly off the dry alcohol topic and more onto the Ethanol from grains; If you wanna make Ethyl Alcohol, so it from sugar, its much easier, and less
likely to have anything else going on. Ive done Potatoes and Barley before, waaaaay less fun than sugar...
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Calcium salts form a goo with ethanol which is a real mess. That is why it is a bad idea to dry ethanol with Ca salts. Big time ethanol producers
now use alumina and/or molecular sieves, which can be dried with steam/heat/vacuum and then reused multiple times. That is the cheapest and simplest
way to dry ethanol.
If you want to make money figure out how to distill ethanol cheaply or even better a non-distillation way to separate ethanol from water. If there
was a way to even remove ~5% of mostly pure ethanol from a 10% ethanol:water mixture, then you could keep brewing non-stop, and then have to heat only
a small volume of ethanol to distill the pure alcohol, which would cut the energy needed drastically. That would compare to the use of reverse
osmosis instead of distillation for the purification of water, which saves a tremendous amount of energy. Alternatively, solar energy could be used
to distill the ethanol, but even that costs a lot in equipment costs.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
You guys are all WAY over thinking this.
I am a distiller. Have been for 2 generations in my family. I followed Gramps's lead.
You can ferment anything w/ simple bakers yeast. On to wheat. There are malt extracts available at any Home Brew Shop or on line. The malt contains
enzymes that convert Starch into complex sugars/ simple sugars, and the yeast ferments these sugars, converting them into Eth.
Expect a 10% return at 100proof or 50%abv. Neither of which will fire an engine. (simple pot still)
If you run your mash thru a fractional/reflux column you will get about 5% return at azeotrope which WILL fire an engine.
Fact of the matter is Eth has more power per volume than Dino fuel, plus it burns cleaner.
Fuel injection needs to be re mapped to run well, and carbs need to be jetted leaner. Mileage therefore is increased.
Brazil is now the world leader in Fuel Eth production. I bring this up to vouch for my point. Any engine can/will run on Eth you can make at home.
Is it viable? Yes... If you have the energy to power a large boiler (55 gallons plus) or if you Vacuum distill (reduced input energy).
I don't know if it appropriate to post links to other forums so I will just state there are a few credible distillation forums, and I belong to one of
them under the same user name.
Thru that forum I have designed a concentric column/mixed media/solar powered water fired boiler that runs under a vacuum assist.
Basically it is a column within a column that produces fuel grade ethanol using solar heated water.
I have some CAD drawings I can attach but I don't know how to "attach" yet.
Short answer is YES, you can run any engine you own on wheat!
Found the attach... This is the boiler / inner rectifying column / outer stripping column. The inner workings are quite simple, but the math to design
it was not.
[Edited on 16-1-2015 by Zombie]
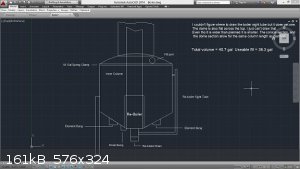
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Mesa
Hazard to Others
  
Posts: 264
Registered: 2-7-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Zombie  | You guys are all WAY over thinking this.
I am a distiller. Have been for 2 generations in my family. I followed Gramps's lead.
You can ferment anything w/ simple bakers yeast. On to wheat. There are malt extracts available at any Home Brew Shop or on line. The malt contains
enzymes that convert Starch into complex sugars/ simple sugars, and the yeast ferments these sugars, converting them into Eth.
Expect a 10% return at 100proof or 50%abv. Neither of which will fire an engine. (simple pot still)
If you run your mash thru a fractional/reflux column you will get about 5% return at azeotrope which WILL fire an engine.
|
Compared to the specific mutant strains being universally advertised among Biotech shops capable of sustaining production of R-OH at near 20%
concentration, it'd be far more worthwhile to spend a half hour researching what you want and ordering it from the labs themselves. I've done it
before and had no issues with staff despite having no ABN(Not owning a registered company... For those outside of Australia.)
| Quote: |
Fact of the matter is Eth has more power per volume than Dino fuel, plus it burns cleaner.
Fuel injection needs to be re mapped to run well, and carbs need to be jetted leaner. Mileage therefore is increased.
Brazil is now the world leader in Fuel Eth production. I bring this up to vouch for my point. Any engine can/will run on Eth you can make at home.
Is it viable? Yes... If you have the energy to power a large boiler (55 gallons plus) or if you Vacuum distill (reduced input energy).
I don't know if it appropriate to post links to other forums so I will just state there are a few credible distillation forums, and I belong to one of
them under the same user name.
Thru that forum I have designed a concentric column/mixed media/solar powered water fired boiler that runs under a vacuum assist.
Basically it is a column within a column that produces fuel grade ethanol using solar heated water.
I have some CAD drawings I can attach but I don't know how to "attach" yet.
Short answer is YES, you can run any engine you own on wheat!
Found the attach... This is the boiler / inner rectifying column / outer stripping column. The inner workings are quite simple, but the math to design
it was not.
[Edited on 16-1-2015 by Zombie] |
[Edited on 20-1-2015 by Mesa]
|
|
|
Luke
Harmless

Posts: 20
Registered: 25-9-2014
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Making alcohol from wheat is easy enough, you will need enzymes to convert the starch in the wheat and then some way of separating the bits left over
from your mash.
You will need a few things to do this on a large scale. A big pot you can heat water in, a plastic 200L drum with a brewing false bottom, a fermenter and some pale ale malt (grain, not the extract) for the enzymes.
Get 35kg of wheat and 7kg pale ale malt (or enzymes if you can get them) and crush it with a mill, dont crush it to a fine flour.
Fit the 200L drum with the false bottom. Add 150L Water @ between 65 and 70 Celcius. Add the grain to the drums and stir it up to make sure theres
no lumps. Final temp should be between 62-66C. If its lower than that youll need to add hot water to bring it above 60C.
Let it sit there for 2 hours and then drain the liquid from the 200L drum to the fermenter. rinse the grains with additional hot water to fill your
fermenter and extract all the sugars. Add yeast to the fermenter (bakers yeast is fine) and allow to ferment at above 25C. This will make a ferment
at about 5% alcohol.
In a week it will be ready for distilling. If you do reading about 'cuts' on homebrew forums youll be able to drink it as well as fuel your car
because you've basically just made a wheat whiskey.
I would mention that instead of using raw wheat use raw barley, it is a lot easier to drain from the 200L drum with false bottom. Edited to add ~
Wheat will basically set like glue. Maybe add some rice hulls (available from all brew shops) to assist with some drainage if you decide to go with
wheat.
I would look at other sources of sugar available to you. In australia we can get bulk molasses for around $200-400 per 1000L molasses. It is about
50% sugar by weight and 1000L weighs 1500kg. So you get about 750kg of sugar for $200-$400
Using something like molasses means you dont have to expend energy doing the mash to convert sugar. PLus your ferment will be a higher alcohol
content meaning less distilling
To ferment molasses just add 40L to 200L water and adjust ph to around 5, add yeast and away you go. This will make a ferment @ around 10%. You
could experiment going higher (to a max of 20%) but i've never done this as it will create off flavours if you are going to convert to drinking
alcohol. Edited to add ~ If you do cuts on you distillation you will make rum, even if using a reflux still.
[Edited on 15-2-2015 by Luke]
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Cool Hand Luke! My man!
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Is there even a modicum of truth in those "20 %" claims?
|
|
|
Luke
Harmless

Posts: 20
Registered: 25-9-2014
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Are you talking about the ability to ferment to 20% alcohol or that doing so makes off flavours?
Bakers yeast can easily get to 15% and turbo yeasts can get to 20%. The high alcohol in a 20% ferment really messes with the yeast though and they
make some terrible flavours that are hard to distill out even when using a reflux still.
If distilling for fuel I'd probably ferment out to 18% or so, just so to not waste any sugars without making the yeast work too hard
There's no way youll get above 12%abv using wheat or barley without wasting lots of grain.
If distilling for drinking i wouldnt go above 14% for rum or 10% for whisky (which would be hard to do achieve using grain alone anyway).
14% for vodka would probably be ok but i usually stick to around 10%
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Luke  |
Are you talking about the ability to ferment to 20% alcohol or that doing so makes off flavours?
|
The former. I like your answer and to the best of my knowledge (amateur brewer) it is correct. Thank you.
|
|
|
| Pages:
1
2 |