0010110
Harmless

Posts: 6
Registered: 16-7-2006
Member Is Offline
Mood: No Mood
|
|
help with diminshed pressure distillation
I have recently obtained a 24/40 ground glass distillation apparatus and most of the stuff i need to distill nitric acid under diminished pressure.
There are a few things im am uncertain of. Don't worry about the safety precautions and handling methods of nitric acid for i am well aware of those;
I have had some experience in handling red fuming nitric acid. The uncertainty i am having is what type of tubing to use when attaching the vacuum
pump to the vacuum outlet on the apparatus. I am unsure if the common clear plastic tubing would with stand the nitric acid + the heat or to long. I
doubt it will. The second thing I am unsure of if the vacuum pump i am using will be sufficient for distilling white nitric acid. The pumps the gauge
average running read out says 25 inches of Hg. I'm not to sure about the type of measurement the gauge uses. Oh and i would also like to mention that
i have two washing flasks to put between the vacuum and the apparatus. Any help would be greatly appreciated and Thank You in advance. 
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Do NOT attach the vacuum adapter to the pump. If you hook directly to your pump you will quickly destroy the seals and rust the guts out of a
mechanical pump distilling nitric acid. Please look up vacuum ditillation in Vogels or Organic Chem Survival Manual, threads here (UTFSE), etc.
There are pressure conversion charts all over the web, use Google. As a minimum you must have a water trap if you use an aspirator or aspirator pump
and you must have a KOH cylinder to protect you mechanical pump from acid. You should have a vapor trap and a vacuum release valve. A balast of 2-3
L is standard and a manometer or guage to assess vacuum, a manostat to control it is nice to have.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
What chemrox said, plus, make sure the hose is thick walled enough as not to collapse under the reduced pressure. Even Tygon type seems to just
barely work in a pinch, but I'd go for something more heavy duty. Butyl rubber car hose might be good. Also, I seem to remember that when distilling
HNO3, you want a small amt of some reducer in the retort. Inevitably, your going to get some NOx near the end, but that doesn't mean that anything is
impure.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Globey, I do not think you want a reducer in the retort. Nitric acid is a strong oxidiser and adding a reducing agent will lead to loss of yield (and
possibly a dangerous scenario). Be aware that (conc.) nitric acid does not like rubber hence the need to distill it in a ground glass setup or
all-glass retort. The only tubing not affected by conc. nitric acid is PFA/FEP/PTFE (consult a chemical resistance chart, there may be others), so use
one of these if possible, otherwise just be careful and dont let your tubing corrode too much.
|
|
|
Contrabasso
Hazard to Others
  
Posts: 277
Registered: 2-4-2008
Member Is Offline
Mood: No Mood
|
|
You need to ensure that the pressure is low enough for the HNO3 to come over without decomposing, you then need to check that the condenser is
adequate to catch all the vapour well before the outlet. Even use two condensers and/or use a small pump to recirculate iced water. Seriously consider
an aspirator pump they are cheaper for when the acid kills them than a mechanical pump.
I have considered getting a bucket of water and a small pump to recirculate through an aspirator pump, but I need to consider whether the pressure
achieved will allow the distilation to procede correctly.
Obviously if the vacuum is too great you will never actually condense the product (it'll go down the pump as vapour) If the vac is too little you risk
the product decomposing.
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
Somebody correct me if I'm wrong. The vapor pressure over a liquid is determined by the temperature of the liquid and vapor at equilibrium. So once
the air , or other non acid vapor, over the boiling acid is removed couldn't you seal the system and maintain the low pressure by adequate cooling on
the receiving side? The only problem would then be pressure resulting from breakdown products of the distillation or pressure rise due to inadequate
cooling. Any thoughts on this?
|
|
|
brew
Hazard to Self
 
Posts: 96
Registered: 25-4-2008
Member Is Offline
Mood: enthralled
|
|
0010110, you could always contact a place that deals with vacuum pumps, used for distilation, as they usualy also sell the tubing that would be the
right strength and size for your needs. 8mm(I.D.)size tubing I believe is the go. Also I'd consider, if this is your beginnings of using vacuum
distillation, start of with something alot safer than Nitric acid. Learn the ins and outs of this type of procedures.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Somebody correct me if I'm wrong. The vapor pressure over a liquid is determined by the temperature of the liquid and vapor at equilibrium. So once
the air , or other non acid vapor, over the boiling acid is removed couldn't you seal the system and maintain the low pressure by adequate cooling on
the receiving side? The only problem would then be pressure resulting from breakdown products of the distillation or pressure rise due to inadequate
cooling. Any thoughts on this? |
That is correct. Once the maximum pressure drop has been achieved equilibrium sets in and the pump is just maintaining the pressure. The bulk of
corrosion occurs during startup, mostly because you are basically degassing your HNO3 first.
If you're planning to do alot of HNO3 distillation a PTFE/ETFE membrane pump might be worthwile. They can be had for $200 used and 100mbar is adequate
for distilling HNO3.
What's inside your washing flasks? Have you provided safeguards against it being sucked into the pump or the apparatus? Note that water vapor is
already capable of destroying a standard rotary vane pump.
[Edited on 23-2-2009 by vulture]
[Edited on 24-2-2009 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@Globey- I'd love to find some of that 2" butyl car hose with a 1/4 or 3/8 id. That is nice stuff for vacuum hookups but I can't find it anywhere.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
| Quote: | Originally posted by Mr. Wizard
Somebody correct me if I'm wrong. The vapor pressure over a liquid is determined by the temperature of the liquid and vapor at equilibrium. So once
the air , or other non acid vapor, over the boiling acid is removed couldn't you seal the system and maintain the low pressure by adequate cooling on
the receiving side? The only problem would then be pressure resulting from breakdown products of the distillation or pressure rise due to inadequate
cooling. Any thoughts on this? |
I think this was misleadingly responded to. In theory this might work, but as you heat your pot of HNO3, its vapor pressure goes up, and the cooling
is not so efficient as to condense 100% of the vapor. In practice, you have to keep pumping on it otherwise the pressure works its way back up to the
point that your distillation stops, whether your distillation be in a rotovap or a fractionation setup.
I would not count on your wash bottles to save your pump. A dry ice trap is pretty standard, but this too will not save it. I'm not familiar with
distilling HNO3, but is an aspirator an option? This would surely save you a lot of troubles.
[Edited on 23-2-2009 by Arrhenius]
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
I'm sure some of my old texts say to use an aspirator.
|
|
|
biotech7
Harmless

Posts: 12
Registered: 27-1-2009
Location: Suzhou City, China
Member Is Offline
Mood: No Mood
|
|
i performed pressure vs B.P. of nitric acid simulation with ACDLab.
i hope it's useful to 0010110.
B.R.
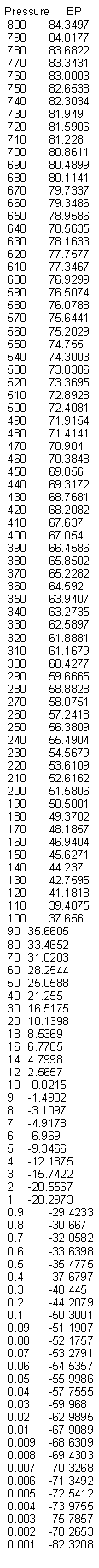
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
There is a nomograph tool on the Sigma Aldrich website you might find useful: http://www.sigmaaldrich.com/chemistry/solvents/learning-cent...
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Mr. Wizard
Somebody correct me if I'm wrong. The vapor pressure over a liquid is determined by the temperature of the liquid and vapor at equilibrium. So once
the air , or other non acid vapor, over the boiling acid is removed couldn't you seal the system and maintain the low pressure by adequate cooling on
the receiving side? The only problem would then be pressure resulting from breakdown products of the distillation or pressure rise due to inadequate
cooling. Any thoughts on this? |
AND the pressure built-up due to ingress of air thru' joints. This is practically unavoidable. Even if the quantity leaked in is very small, at low
pressures it results in significant volumetric load on the pump.
gsd
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Oh ya, you definitely need an aspirator. You just need to get below the point where the acid significantly decomposes, right?? Should get you there,
and there's no need for traps etc. 
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
You still need a trap to prevent suckback of water into your freshly distilled conc. nitric acid. Otherwise you can have a potentially dangerous
situation on your hands. Some aspirators have "check valves" but I would still put in a trap to be safe.
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Often those valves are unreliable!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Finding out it fails in the middle of a nitric acid distillation is definately not good timing, hence the suggestion for a trap 
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Ahh, oui oui... trap for water. I threw a gauge on an aspirator today and got down to 400torr with ease, which is sufficient I should think.
|
|
|
0010110
Harmless

Posts: 6
Registered: 16-7-2006
Member Is Offline
Mood: No Mood
|
|
I appreciate all the advice. I think I have decided to just purchase a couple small plastic water jet aspirators. I really don't think my mechanical
pump is such a good idea. I tried a few vacuum distillations with just water and the pump seemed to be having a hard time keeping a vacuum. it
constantly jumped from high vac to low. there was no leak in the system so i figured the pump just isn't cut out for it.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by 0010110
I really don't think my mechanical pump is such a good idea. I tried a few vacuum distillations with just water and the pump seemed to be having a
hard time keeping a vacuum. it constantly jumped from high vac to low. there was no leak in the system so i figured the pump just isn't cut out for
it. |
The leak is in the pump. It's burping, is my guess, although it's hard to say much for certain without
knowing what kind of pump. Although even from what you've said, I'd worry about whether your oil level is adequate and whether the oil has sufficient
viscosity (either from being old or being the wrong kind of oil).
|
|
|