| Pages:
1
..
22
23
24
25
26
..
30 |
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by sonogashira  | ...
Preferably I would like to separate the benzaldehyde from the DCM-Acetophenone-Benzaldehyde. I thought maybe bisulfite can work but I think
acetophenone will react as well.
I gather you do not think it (benzaldehyde) can be removed just with water washing of the DCM solution? Or by heating above the bp of benzaldehyde?...
|
Check the relative solubilities of the two products
acetophenone 5.5 g/L at 25°C, 12.2 g/L at 80°C
benzaldehyde 0.6 g/100 ml (20 °C)
so you're looking at a ratio on the order of 9:10, which means counter current extraction a best, and multiple stages most likely.
You may want want to get the catalyst out of solution first, or extract them away from the aqueous phase, before attempting any further workup.
Matter of fact, doesn't this take a cosolvent or emulsifier to get good results?
Bisulfite will work, the aromatic ketones ArC=O... do not add bisulfite very well, even the methyl ketone acetophenone. You'll have to experiment a
bit, basic idea is to wash the organic phase with nearly saturated NaHSO3 solution one or more times. Check each phase, the aqueous one after
destroying the addition product and extracting the organics back into DCM; perhaps use TLC to evaluate the separation.
You'll end up with a little of the ketone coming along with the aldehyde, and aldehyde with the ketone. Use oxidation to clean up the ketone, a
water-acetone solution of it and drip in KMnO4 solution persists for 10s of seconds, then add a ml or 2 of isopropanol to destroy the small excess of
KMnO4. Filter off "MnO2", distill off acetone, add NaHCO3, extract ketone out with DCM.
Note that there will likely be unreacted styrene and some polymers of it. If you distill the acetophenone add a chaser to it before distilling, some
non-reactive substance it mixes with and that boils much higher than the ketone. Kerosene that has had its low boiling components removed in a
fractionation (just fractionate out the low boilers, don't distill the high boiling stuff you want) works well. The chaser allows most to all of the
acetophenone to be fractionated out without running the still pot dry, and any styrene polymers will dissolve in the hot chaser - easier to remove
from the flask.
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Hey, thanks very much! You're right, there will be a small amount of acetone co-solvent (few ml), but it should be removed with the water wash. Sodium
lauryl sulfate apparently converts 100% of styrene and with 100% acetophenone selectivity (zero benzaldehyde) - but it is claimed that it "forms an
emulsion which is very difficult to separate."
[In fact, any good ideas to separate this and palladium chloride from the acetophenone product would be very useful also! This method may be even more
useful if there is an easy way to separate them which the authors overlooked!]
Yes, acetophenone does tend to help polymerization - this is the reason I want it, in fact  - so maybe I will need to distill. Probably I could use petroleum jelly (vaseline) as 'hot chaser' to save distilling kerosene... - so maybe I will need to distill. Probably I could use petroleum jelly (vaseline) as 'hot chaser' to save distilling kerosene...
Thanks for all the tips!  I wanted to be sure I could purify the acetophenone
before I waste money on buying the palladium chloride! I wanted to be sure I could purify the acetophenone
before I waste money on buying the palladium chloride!
[Edit: and if the bisulfite won't remove all of the benzaldehyde maybe I will just go straight to oxidation to benzoic acid instead - I thought I
would add this incase anyone can see a problem with the method I intend.]
I may even try distillation of calcium salts also and compare the two processes - but first to get a distillation set! 
Second edit: according to US4433173 diethylene glycol is the solvent to use. It increases the volatility of the contaminants and one can then boil-off
the acetophenone from the (non-azeotrope) mixture of the two - Diethylene glycol having relative volatility of 0.1 compared with acetophenone. I think
this is the implication, but I will have to read it a few times more to make sure I understand correctly because of all the silly language they use
for some ridiculous reason 
[Edited on 2-1-2010 by sonogashira]
[Edited on 2-1-2010 by sonogashira]
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Quote: Originally posted by sonogashira  | Sodium lauryl sulfate apparently converts 100% of styrene and with 100% acetophenone selectivity (zero benzaldehyde) - but it is claimed that it
"forms an emulsion which is very difficult to separate."
[In fact, any good ideas to separate this and palladium chloride from the acetophenone product would be very useful also! This method may be even more
useful if there is an easy way to separate them which the authors overlooked!]
[Edited on 2-1-2010 by sonogashira] |
Time separates all emulsions, however if you are time poor my most reliable technique is the microwave, play with power levels and times, it can break
an emulsion within seconds at times. If i have no joy with the microwave i use the freezer, freezes the water out, then just invert your flask onto a
course filter and leave the organic solvent to drip out. This is all done in the freezer of course (not the microwaving though)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The bisulfite will remove the majority of the aldehyde, but a small amount will remain unless you push things with several extractions with aqueous
bisulfite. However as the ketone is slightly water soluble a bit will be leached out into the aqueous layer with each extraction. As it is easier to
handle traces of aldehyde in ketone as opposed to the reverse, it seems better to leave a bit of aldehyde behind and scrub it out as the acid.
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Thank you Panache - unfortunately in this case the freezing point of acetophenone is 20 C (although when pure it is typically liquid at much lower
temperature - unless there is impurities.) It will be worth to try and I do have lots of time, so I shall try (with a test-sample) to microwave, and
also leave it standing.
not_important - I do not want to be a pain but there is one thing I don't understand: why use the bisulfite when I will need to use KMnO4 anyway?
Could I not oxidize all of the benzaldehyde (10% benzaldehyde and 90% acetophenone) after removing the DCM? Maybe by adding water and acetone with
KMnO4, as you suggested? (Maybe acid is needed too? - I have rarely used KMnO4 for organic oxidations... But I believe base and KMnO4 may turn
acetophenone into benzoic acid?)
I want to prepare this (acetophenone) as solvent for some polymerization reactions, so I do not mind if this would cause extra expense with the
purification step - I just want it as pure as possible. Is there some benefit in using bisulfite?
Sorry to be a bother, and thank you (both) for the kind help! 
[Edited on 2-1-2010 by sonogashira]
[Edited on 2-1-2010 by sonogashira]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Well, because you said that you wanted to recover both if you could :-)
If you're just cleaning up traces you can 'titrate' with the KMnO4, adding it drop by drop until the colour remains for some seconds; little chanch of
chewing up much of the ketone with a dilute solution. The reaction rates of aldehydes and ketones to KMnO4 are generally much different, so if you
don't just dump in gobs of the oxidiser you can readily tell when the aldehyde has been used up without attacking the ketone.
Or you can use one of the other redox reactions that aldehydes undergo readily while ketones do not, acidified dichromate - not too concentrated,
Fehling's or Benedict's solution, or:
using Oxone http://www.organic-chemistry.org/abstracts/literature/565.sh...
using sodium perborate+acetic acid http://www.organic-chemistry.org/abstracts/literature/266.sh...
those last two might be useful if you just want to skip the separation and instead oxidise away all the aldehyde as the reagents are fairly cheap, low
toxicity, and don't stain stuff.
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Oh I see  Maybe I implied that - but really I meant the exact opposite! I thought
perhaps you knew of some obscure side reaction which I hadn't considered! Anyway, nevermind... Maybe I implied that - but really I meant the exact opposite! I thought
perhaps you knew of some obscure side reaction which I hadn't considered! Anyway, nevermind...
Thanks very much for the help. I will look into appropriate oxidizer to use... and hope for the stated yields!
Best wishes
[Edited on 2-1-2010 by sonogashira]
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
I am considering the potential effects of water on the chlorination of toluene in the presence of tin(IV) chloride. The catalyst is soluble in
toluene, but would water interfere with the interaction of catalyst and substrate?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by manimal  | | I am considering the potential effects of water on the chlorination of toluene in the presence of tin(IV) chloride. The catalyst is soluble in
toluene, but would water interfere with the interaction of catalyst and substrate? |
Water will not interfere with the electrophilic chlorination itself, but it will decompose the catalyst by hydrolysing it (SnCl4 + H2O => SnO2 +
HCl). So you do not want water in there when using hydrolysable Lewis acids as catalysts.
When using NCS, trichloroisocyanuric acid or other chloroimides instead of Cl2, you can use protic acids as catalysts, and in this case the presence
of moisture does not interfere much or not at all (in fact the reaction can even be run with water in a biphasic system).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Quote: Originally posted by Nicodem  | Water will not interfere with the electrophilic chlorination itself, but it will decompose the catalyst by hydrolysing it (SnCl4 + H2O => SnO2 +
HCl). So you do not want water in there when using hydrolysable Lewis acids as catalysts.
When using NCS, trichloroisocyanuric acid or other chloroimides instead of Cl2, you can use protic acids as catalysts, and in this case the presence
of moisture does not interfere much or not at all (in fact the reaction can even be run with water in a biphasic system).
|
Thanks. What I had in mind to do, was to add the entire measure of chlorinator (TCCA) to toluene and catalyst, followed by a small (catalytic)
quantity of HCl. This would introduce chlorine in a rate-limiting manner as C3Cl3N3O3 + 3 HCl + 3 C6H5CH3 --> C3N3H3O3 + 3 C6H4ClCH3 + 3 HCl. Thus,
the initial Cl2 formed would have to react with the toluene first, releasing HCl which would be re-oxidized to Cl2 (in lieu of adding chlorine or
oxidizer gradually over a period of time).
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by manimal  | | Thanks. What I had in mind to do, was to add the entire measure of chlorinator (TCCA) to toluene and catalyst, followed by a small (catalytic)
quantity of HCl. This would introduce chlorine in a rate-limiting manner as C3Cl3N3O3 + 3 HCl + 3 C6H5CH3 --> C3N3H3O3 + 3 C6H4ClCH3 + 3 HCl. Thus,
the initial Cl2 formed would have to react with the toluene first, releasing HCl which would be re-oxidized to Cl2 (in lieu of adding chlorine or
oxidizer gradually over a period of time). |
What you propose does not sound particularly rational chemistry-wise. Why using TCCA as oxidant when you can chlorinate toluene directly and even
considerably more efficiently by using it as the electrophile?
Just add a couple of mol% of tosylic acid monohydrate, AlCl3, FeCl3, SnCl4 or any other strong enough acid to a solution of TCCA in excess toluene and
let stir overnight, filter off the cyanuric acid and fractionate the filtrate. Or just follow the literature example as done by Axt for chlorination
of benzene in this thread. Don't forget that electrophilic halogenations of toluene are quite exothermic so never add too much acid catalyst and never do the
first trial above about 10 mmol. TCCA in the presence of acid catalysts is more electrophilic than Cl2 which reacts with toluene quite sluggishly, so
take this into account when planing anything on a larger scale.
Are you planing to separate the ortho- and para-chlorotoluenes and if so how?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Quote: Originally posted by Nicodem  | What you propose does not sound particularly rational chemistry-wise. Why using TCCA as oxidant when you can chlorinate toluene directly and even
considerably more efficiently by using it as the electrophile?
Just add a couple of mol% of tosylic acid monohydrate, AlCl3, FeCl3, SnCl4 or any other strong enough acid to a solution of TCCA in excess toluene and
let stir overnight, filter off the cyanuric acid and fractionate the filtrate. Or just follow the literature example as done by Axt for chlorination
of benzene in this thread. Don't forget that electrophilic halogenations of toluene are quite exothermic so never add too much acid catalyst and never do the
first trial above about 10 mmol. TCCA in the presence of acid catalysts is more electrophilic than Cl2 which reacts with toluene quite sluggishly, so
take this into account when planing anything on a larger scale.
Are you planing to separate the ortho- and para-chlorotoluenes and if so how? |
Actually, I was going by US3000975 (http://www.google.com/patents?id=V6tJAAAAEBAJ) on the chlorination of toluene. It specifies quite high selectivities of >95% and a preference
for the o-isomer (75/25%) with chlorine gas in the presence of a tin chloride catalyst.
I considered the use of TCCA directly w/H2SO4, but was put off by the statement that it carries yields of only 66% (with preference for the p-isomer)
according to TCCA - A Safe and Efficient Oxidant. I realize the patent might be garnishing its claims, and may indeed yield no higher than
66% either. I'm not particularly set on separating the isomers, but rather just to see how the chlorination goes.
[Edited on 22-1-2010 by manimal]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I'm quite sceptical about that patent. Chlorination of toluene with Cl2 almost invariably gives a near to statistical ratio of ortho vs. para
chlorotoluene (which would be 67% : 33%). Sometimes the ratio is less, but I don't remember ever seing examples where it would be less then 50%
ortho-chlorotulene unless electrophiles other than Cl2 were used or under some special conditions. A 75:25 ratio is out of the statistical
distribution which means there must be some directional effect from the catalyst. If this would be the case then SnCl4 would be known for it and there
should be some paper about it (and not just a claim in a patent). Obviously if you follow that patent you need to introduce dry Cl2 from outside
reaction. Adding TCCA in the presence of SnCl4 would just cause direct chlorination from this reagent instead of Cl2 while adding HCl(aq) would quench
SnCl4. In either case you would not get the desired ortho regioselectivity increase (provided that the patent claim is true).
The claim that under such biphasic conditions TCCA gives 66% para regioisomer is also a bit uncertain. If you check the method used to determine the
para/ortho ratio in the original paper (JOC, 35, 719–722), they did so by measuring the refractive index. This property is highly sensitive
for any contamination of other compounds (residues of toluene, polychlorotoluenes, etc.) and I would not trust that data with much accuracy. They did
not use GC like they should. I would expect that under such conditions one gets a near to 1:1 mixture of ortho- vs. para-chlorotoluene.
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Quote: Originally posted by Nicodem  | Chlorination of toluene with Cl2 almost invariably gives a near to statistical ratio of ortho vs. para chlorotoluene (which would be 67% : 33%).
|
That's interesting. I was perusing the old literature and it said that iodine is a ring-chlorination catalyst (presumably by forming iodine
trichloride which acts as electrophile) which favors almost exclusively the p-isomer. However, many more recent patents have been filed for increasing
p/o ratio with wacky catalysts like ferrocene, which barely achieve 50:50. Why would they go to such trouble if catalytic iodine can facilitate it
much more easily? Perhaps the early lit. references were mistaken.
[Edited on 23-1-2010 by manimal]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by manimal  | | That's interesting. I was perusing the old literature and it said that iodine is a ring-chlorination catalyst (presumably by forming iodine
trichloride which acts as electrophile) which favors almost exclusively the p-isomer. However, many more recent patents have been filed for increasing
p/o ratio with wacky catalysts like ferrocene, which barely achieve 50:50. Why would they go to such trouble if catalytic iodine can facilitate it
much more easily? Perhaps the early lit. references were mistaken. |
That iodine catalyses the chlorination is no surprise as it is after all a Lewis acid, but I never heard about its influence on the regioselectivity
of toluene chlorination. I would really like to read more about it. Could you please dig out the reference describing all this?
|
|
|
Sobrero
Harmless

Posts: 21
Registered: 20-5-2006
Location: Belgium
Member Is Offline
Mood: Lifting my skinny fists like antennas to heaven
|
|
What is this piece of glassware used for? I can't think of any setup where this would come in handy.
(It makes a good bong though  ) )

"There exists a world. In terms of probability, this borders on the impossible." (Jostein Gaarder)
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
The spheres might be for drying agents. Don't ask how you're supposed to put the stuff inside them..
Tim
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
what is name of this componet?
this componet is possible?if yes how we can make it?
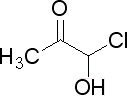
Chemistry=Chem+ is+ Try
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Acetone chlorohydrin.
Looks unstable, and certainly irritating. I don't recommend making it.
Tim
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
@Sobrero
I dont know much about glassware but suck back traps perhaps?
Seems like something I would love to have right now to feed NH3 into Ether and use the Bulbs as suckback traps.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
cadra
Harmless

Posts: 1
Registered: 27-1-2010
Member Is Offline
Mood: No Mood
|
|
Hi Everyone, this is my first post. I didn't know whether to post in short questions or someplace else. I have what I hope are a couple of quick
questions for someone with more experience than me.
I have a Schiff base that I would like to methylate. I know that I can do this via a number of methylating agents such as dimethyl sulfate, methyl
tosylate, or MeBr. However, pure curiosity has lead me to wonder if I can also methylate a Schiff base via Eschweiler Clarke reaction. After
methylation I would like to hydrolyze to hopefully provide a mono-N-methylated amine and the starting aldehyde.
My first question is: Does this seem possible? Has anyone heard of this (I can't seem to find any references in my searches).
My second question: If this is possible, I am fearful that if I utilize oxalic acid dihydrate and paraformaldehyde to effect the Eschweiler Clarke as
discussed in this thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=7263
that the water from the dihydrate will hydrolyze the Schiff base prior to methylation, causing dimethylation of the amine (which is not what I want).
Here's another thread that I found on monomethylation of amines where the method was presented briefly but I didn't see anyone trying this on a Schiff
base such as could be made by condensation of benzaldehyde with amine:
http://www.sciencemadness.org/talk/viewthread.php?tid=8375#p...
Note that to perform the Eschweiler Clarke utilizing oxalic acid dihydate and paraformaldehyde in the above mentioned paper, tempuratures of 100-120C
need to be used to decompose oxalic acid to formic acid.
Thank you very much in advance!
Cadra
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
?
Does anyone know a good method for capturing CO.
The reaction I am looking at produces certain amounts of it and yeah as we all know CO is a bitch.
So can anyone suggest an absorbent/reactant to destroy/react it?
Thnx
What a fine day for chemistry this is.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
| Quote: | | Does anyone know a good method for capturing CO. |
One of my lab manuals gives a recipe for a CO abosrber:
Dissolve 200 gm cuprous chloride and 250 gm NH4Cl in 700 mL of water and add 1/3 its volume of conc. NH4OH.
The lab manual is Introduction to Organic Laboratory Techniques by Pavia, Lampman and Kriz.
Roscoe's Treatise of Chemistry confirms that CO is soluble in ammoniacal cuprous chloride.
When I use CO I keep a CO detector running on the bench to be sure the hood is capturing it all.
[Edited on 30-1-2010 by entropy51]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
This has been discussed at length before, about 2 years ago, you'll need to search the forum.
Hopcalite was used at one time in respirators - 50%MnO2(I assume the active form) copper oxide (30%) cobaltic oxide (15%) and silver oxide (5%).
Or you could bubble the CO through blood 
"Destroying carbon monoxide in a gas stream"
The thread is here : http://www.sciencemadness.org/talk/viewthread.php?tid=10394#...
[Edited on 30-1-2010 by Xenoid]
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
What is a reasonable price for a second hand heating mantle?
Electromantel 100ml max 450 deg C.
Price 250 euro's
Isopad 250 ml , not temp specified.
price 250 euro's
Its seems like a rip off to me..
I am having a hard time finding a good deal for these devices.
And I dont do ebay, often the shipping is more than the original price.
*edit*
I mailed the company that had these offers.
I said that i found the prices where just too high.
Really laughing my ass off now i got a reaction.
The new the proposed price is 200 euro's for a model that costed 360.
Its a model with build-in stirrer.
It's still too much money for my liking but maybe iam not rational.
Still funny though.
[Edited on 4-2-2010 by User]
What a fine day for chemistry this is.
|
|
|
| Pages:
1
..
22
23
24
25
26
..
30 |