thysonsacclaim
Harmless

Posts: 5
Registered: 6-7-2009
Member Is Offline
Mood: No Mood
|
|
Problems setting up steam distillation apparatus
I am new to chemistry (have only taken 2 college-level courses) and was looking into something a little more involved than simply mixing and filtering
chemicals. The most complicated things I have done to this part are the syntheses of alum and purple nickel compounds.
I finally decided on putting together a steam distillation apparatus because it is relatively safe for me to start out with and will allow me to have
usable products which will have little risk for harm (namely, essential oils from oranges, mints, etc).
After researching a bit, I found some issues I could not get definitive answers to:
So far I have an Erlenmeyer, lengths of 5mm glass tubes, a Liebig condenser (24/40 ST on one end, fitted for stopper on the other end), a burner, a
collection flask and most of the appropriate stoppers.
The Erlenmeyer flask will be used for steam generation, which will then be pumped into a distillation flask containing the organic material where it
will then be forced through the condenser. There is a picture of a similar apparatus here.
Problems: I can't find glass tubes longer than 24 inches for connecting the steam generating E. flask to the distillation flask. Is it appropriate to
use amber latex hose to connect multiple glass tubes together (the type found at UnitedNuclear.com)?
Also, since getting a Liebig condenser with 24/40 joints on both ends and the appropriately sized distilling flask with a 24/40 adapter to connect to
it was out of my price range, I opted instead to go with a condenser that can be stoppered.
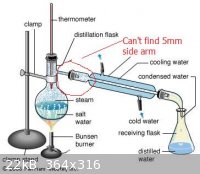
I was hoping I could stopper it with a single-holed stopper and connect a distillation flask with a side arm (such as shown here) to it via the hole. However, most distillation flasks with side arms I saw only showed the inner diameter and I had trouble finding one with
a 5 mm side arm to fit inside the stopper. I also couldn't find stoppers with something besides 5 mm holes. How would I do this? I have attached a
picture of this area for visual reference (although it is of simple distillation).
Thanks
Scientia est potentia
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
You can get longer tubing online, for example http://www.winshipdesigns.com/home/ws3/smartlist_70/medium_w...
Also glass artist suppliers are worth checking, as are local glass art/bong stores - they may sell you a single piece if they use that size.
Soft/flint glass may be more difficult to find, and takes a hotter tourch to work.
Latex tubing should work if you butt the glass pieces together. High temperature tubing, such as used in automobiles, is better if you can find a
size match, silicone tubing is another option.
You can use a decent quality cork stopper on the condenser if you allow the side tube of the flask to protrude fairly far into the condenser's flared
end, to keep the hot vapours away from the cork.
As an alternative, if just focusing on essential oils then all-metal apparatus can be fabricated and used.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
You'll need a ringstand and a ceramic coated wire gauze unless you want to break your flask in short order. Open flame is a massive no-no. Hotplates
are much preferred to fire, but are pricey in comparison. Then again, if you break a lot of glass with the flame, the hot plate may be cheaper after
all.
It should be fairly easy to find a set of stopper punches for a few dollars. These can be used to enlarge smaller holes or punch holes in solid
stoppers.
Don't you mean borosilicate takes a hotter flame?
[Edited on 7-7-09 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
thysonsacclaim
Harmless

Posts: 5
Registered: 6-7-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for the info! It is just essential oils for now, but I will surely use it for other things. I am loving my chemistry courses. 
I can't afford a hot plate just yet. I was planning on using an alcohol burner burning 91% isopropanol or denatured ethanol. Would a burner such as on
a smooth [glass] top electric stove be, ok in lieu of the flame?
[Edited on 7-7-2009 by thysonsacclaim]
Scientia est potentia
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
If using a burner then ideally you will also use a gauze to "disperse" the flame and make heating less localised. Another option is to add the
material to be extracted to a distilling flask, with water, and distilled normally. The essential oils also come over with the steam, and separate out
after being condensed. I know this works for certain when extracting limonene from orange peel.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by thysonsacclaim  | Thanks for the info! It is just essential oils for now, but I will surely use it for other things. I am loving my chemistry courses. 
I can't afford a hot plate just yet. I was planning on using an alcohol burner burning 91% isopropanol or denatured ethanol. Would a burner such as on
a smooth [glass] top electric stove be, ok in lieu of the flame?
[Edited on 7-7-2009 by thysonsacclaim] |
An oil bath is much more convenient for this kind of work. I improvised one out of by electric frypan, but deep-fryer would have been even better.
Later, I bought a gallon of glycerol to replace the vegetable oil that I was using.... much easier to clean the outside of the flasks now.
You won't get nearly enough heat from an alcohol burner, and heat transfer from a hotplate is poor.... but you will need both these for other projects
if you pursue your interests. You'll also need a decent bunsen burner and gas supply .... propane maybe.
|
|
|
thysonsacclaim
Harmless

Posts: 5
Registered: 6-7-2009
Member Is Offline
Mood: No Mood
|
|
I figured I might have a problem with the alcohol burner. I don't have the means for a complete Bunsen setup either. How about a small butane burner?
Scientia est potentia
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Depends on the scale you intend to work. And what do you mean by "small"? A butane blowtorch (such as those used for the soldering of copper plumbing
pipes) should be sufficient for... well, I dont know exactly, but I wouldnt use one for anything more than about a liter of water?
|
|
|
1281371269
Hazard to Others
  
Posts: 312
Registered: 15-5-2009
Member Is Offline
|
|
A bunsen set up is not too difficult, all you need is a cylinder of gas, a regulator, a bunsen and the relevant tubing. If you only bought a small
cylinder I expect that together they might even cost less than a hot plate.
A great advantage of using oil baths: All your experiments smell of chips 
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
I found a relatively cheap way to get a Bunsen (assuming you don't already have a gas hookup).
It's a BernzOmatic JTH7 high temperature torch that I found in the local hardware, for about $40 IIRC.
It's a hand-held torch that's essentially a bunsen burner connected to a regulator for a small propane tank by about 3 feet of high pressure tubing.
Clamp the hand-held burner to a ringstand and it functions as a very good Bunsen. Unlike a torch connected directly to a propane cylinder, there's
little risk of tipping it over and starting a fire.
|
|
|
jmneissa
Harmless

Posts: 29
Registered: 4-5-2009
Member Is Offline
Mood: No Mood
|
|
hey guys I have been trying to distill essential oils for the past couple of days with no success. I have mainly been using lavender from my garden. I
am a amateur chemist who has done distillations before but not essential oil ones. Well anyway I used the flowers of the lavender plant add a little
water to them ground them up a little and then poured into a 500ml round bottom flask. I distilled for an hour and a half and a liquid came over when
I held it up to the light I could see no oil layer. I mixed with a saturated sodium chloride solution. This did nothing. I followed the same procedure
as seen Here. Except they got oil and I didn't. Now I used slightly less lavender to start with but everything else was the same. Hopefully one of you
guys can tell me whats up..
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
How old is your lavender? The age of them may play a roll if its oils are volatile.
ry passing steam into the lavender while boiling away the water that forms.
I've got the oils out of sage by blowing steam into a distillation set up, while heating it on an oil bath to boil away any of the formed water. I got
a layer of oil, and the water was also scented.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Other factors that play a role in essential oil yield includes the time of collection, with morning after most of the dew has evaporated but before
the day heats up much.
The particular species of lavender is also important, some types contain little oil. For the types used commercially, the yield is about 1 liter per
30 kg of plant; lower yielding plants can result in a single water layer that will carry a lavender scent.
|
|
|