Mephisto
Chemicus Diabolicus
  
Posts: 294
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
Picramic acid: reduction with thioacetamide
I want to discuss here the possibility of a theoretic synthesis, thought up by myself. For better understanding I will compare my idea with a
synthesis-method taken from “Fundamental processes of dye chemistry” (by Hans Eduard Fierz-David and Louis Blangey, New York: Interscience
Publishers, 1949). Here the original method:
[color=darkred]For example, 0.6 mole (137.5 grams) of picric acid is mixed with 1.2 liters of water and 36 grams of soda ash at 50°. Solution is not
complete. When the carbon dioxide has been expelled, a solution of 1 mole (240 grams) of crystalline sodium sulfide in 450 cc. water is added, with
good stirring, during the course of 30 minutes. Simultaneously, a mixture of 108 grams of 30 per cent hydrochloric acid and 300 cc. water is added at
such a rate that this addition requires about 1 minute longer than that of the sodium sulfide solution. Stirring is then continued, without heating,
for 30 minutes, and the mixture is filtered after 12 hours. The precipitate is washed with 100 cc. saturated salt solution. This crude sodium
picramate is dissolved in 2 liters of water, and the solution is filtered and poured into a hot (90°) solution of 70 cc. 30 per cent hydrochloric
acid in 400 cc. water. The pure picramic acid is precipitated completely after 24 hours, and is filtered off, washed with a small amount of water, and
dried at 80°. The yield is about 100 grams, or 83 per cent of the theoretical amount.
4 X-NO<sub>2</sub> + 6 Na<sub>2</sub>S + 7 H<sub>2</sub>O --> 4 X-NH<sub>2</sub> + 6 NaOH + 3
Na<sub>2</sub>S<sub>2</sub>O<sub>3</sub> (X=Dinitrophenol) [/color]
My idea based on the reduction of picric acid with thioacetamide. Here the equation:
4 X-NO<sub>2</sub> + 6 C<sub>2</sub>H<sub>5</sub>NS + 13 H<sub>2</sub>O --> 4
X-NH<sub>2</sub> + 6 NH<sub>4</sub><sup>+</sup> + 6 CH<sub>3</sub>COOH + 3
S<sub>2</sub>O<sub>3</sub> <sup>2-</sup>
For those, who don’t know thioacetamide. This compound is used as a substitute for H2S in laboratory qualitative analyses. It’s not OTC. It reacts
with hot water to H2S, ammonia and acetic acid.
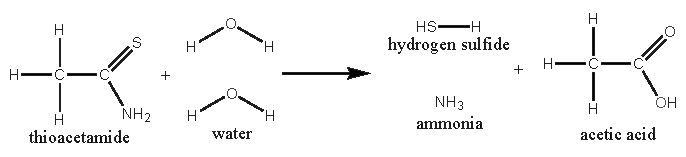
So the picric acid should be dissolved in hot water. Thioacetamide should be added (ratio picric acid : thioacetamide = 4 moles : 6 moles). After
heating, the solution would be red. The precipitated picramic acid could be filtered off from the chilled solution.
Is the presence of sodium carbonate necessary? I think it isn’t necessary in the original method (too), but it is used (why?).
Please, tell me your arguments and opinions if this reaction could work fine. I will test it sometime and post the results.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I presume the soda is there to get some of the picric acid into solution and to buffer the pH so you don't just get Na picrate and H2S.
Presumably the acid is added along with the sulphide to keep the pH reasonably constant. IIRC this reduction is also done using sodium
hydrogensulphide (NaHS) so I guess that is some clue to the "best" pH. The thioacetamide looks like it might work provided that the NH3 and
CH3COOH don't upset the pH.
Given that thioacetamide isn't an OTC chemical and it is a carcinogen I'm not sure this synthesis will get used much. Pity, it's more
interesting than most.
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 294
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
Yes a pH buffer. Sounds logically.
I think I can test the procedure in a few free hours till New Year with some old picric acid. Then we should know more.
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 294
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
Seems not to work
I have tested my method and it seems not to work. Here a short description of what I have done:
1. I mixed 15 grams of dry picric acid with 7.9 grams sodium carbonate in 200 ml of water. (Yes, I decided to take a higher
water/picric acid ratio than in the original method.)
2. I heated the solution to 90 degrees and added a second solution of 7.4 grams thioacetamide in 75 ml water under continually
magnetic stirring. (So picric acid and thioacetamide are mixed in a stoichiometric ratio. The temperature of the picric acid solution is higher as in
the original method, because the thioacetamide decomposes only at such high temperatures to H2S, ammonia and acetic acid.)
3. The colour of the solution didn't change; a smell of H2S was present. After 10 minutes I added a solution of 20.5 ml 30% HCl
in 65 ml water. Yellow crystals precipitated and it wasn't possible to stir the solution/crystal-cake.
4. After an additional hour I filtered the crystals off. They were still yellow. I didn't continued the process and dried the
precipitation.
I think picramic acid and its salts are red and not yellow. Actually the precipitation should be red sodium picramate. After drying I will see, if
there is a difference between my yellow intermediate product and picric acid, but now I think the picric acid precipitated without having react with
the thioacetamide.
Note: I know that patience is the most important thing in chemistry. But I hadn't 12 hours time to let this bad smelling solution stand (and it
was to cold outside to let it stand outside or keep 12 hours the window open).
At least on the paper this method looks fine.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Your problem with the reduction is likely pH related. An excess of ammonia is needed or an alkaline condition is required for the hydrolysis of the
thioacetamide, (in order for an alkaline sulfide to be produced), which is the *required* reductant for the alkaline picrate. In your
reaction the ammonia is tied up as ammonium acetate, and there is not present the excess ammonia needed to neutralize the H2S to form the needed
ammonium sulfide which would then accomplish the reduction. The attached article will shed some light on this.
[Edited on 22-2-2009 by Rosco Bodine]
Attachment: American_Journal_of_Science 1861 pg188.pdf (145kB)
This file has been downloaded 939 times
|
|
|
Mephisto
Chemicus Diabolicus
  
Posts: 294
Registered: 24-8-2002
Location: Germany
Member Is Offline
Mood: swinging
|
|
That's over 5 years ago. Amazing how fast time passes  . Many thanks for your
research work Rosco to solve this thread! . Many thanks for your
research work Rosco to solve this thread!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Sorry for the belated reply  better late than never better late than never 
Just happened to stumble across this "cold case file" and
it was too good to pass up.
Don't be a stranger .
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Maybe the use of thiourea in basic media would be as good or even better than thioacetamide ...?
H2N-CS-NH2 + 2H2O --> H2S + CO2 +2NH3
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It would seem so. Doesn't this stuff really take us way back, these sulfur compounds and their often potentially odorous reaction products .....it
just smells like some real chemistry is afoot, not the anorexic clinical chemistry of instruments and dials ....but an old days sort of chemistry
experiment having some meat on the bones  It is good It is good
once in awhile to stink up a beaker, while stopping short
of causing the local birds to fall from the sky
|
|
|