Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
How does bromination catalyzed by red phosphorus work?
We all know that a-bromination of acids can occur using bromine and catalytic amounts of red P, as described in the preparation of bromoacetic acid. Most of use know that PBr3 is formed in-situ, reacting with acetic acid to form acetyl bromide, which is
then easily a-brominated. But the PBr3 can only participate in one catalytic cycle, as it is transformed to H3PO3.. So the formed a-bromoacetyl
bromide reacts with AcOH to form bromoacetic acid and acetyl bromide, etc etc..
But looking at the notes from the above preparation, I noticed this statement:
"[bromoacetic acid has been prepared] with red phosphorus as a catalyst with the formation of bromoacetyl bromide"
and
"Acetic acid has been converted into bromoacetyl bromide by action of bromine in the presence of red phosphorus,"
So how can this work? How can it be possible the brominated acyl bromine is obtained while using only catalytic P? What is the PBr3 turned to?
In the first ref of this document, a french document ( Ann. Chim. Phys. 17, 83 (1879) details the preparation of acetyl bromide from 1eq bromine added to 1eq of GAA
in presence of roughly 10% red P.. They obtain 1500g of acetyl bromide from 750g of GAA!
This just doesn't add up.. Where does that oxygen go?
AcOH + Br2 --red P--> AcBr + HOBr?
then what, HOBr --> HBr + 1/2O2?
Sounds pretty skechy to me.. But the experiemental is there! So that would be mean that by adding 2 eqs of bromine, one could form bromoacetylbromide,
right? Thus verifying the claim :
"Acetic acid has been converted into bromoacetyl bromide by action of bromine in the presence of red phosphorus,"
Also, this document in interesting:
http://books.google.fr/books?id=x77djyQHX8UC&pg=PA193&am... (page 193)
To 15mL (0,25mol) of GAA containing 1,5g red P (0,05mol) they add 10mL bromine (0,20mol), and they distill bromoacetyl bromide.. No yields are
stated, so here thr red P might simply act as a source of PBR3..
SO I'm stuck with this delema.. Any ideas?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
I don't think the phosphorous acts as a catalyst. Maybe it catalyzes the reaction (in terms of speed), but it is not a "true" catalyst since it is not
regenerated. Perhaps the phrase is not meant to be taken too literally?
I'm not sure if that is what you were asking but at least I tried!
[Edited on 19-9-2009 by sonogashira]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Quote: Originally posted by Klute  |
To 15mL (0,25mol) of GAA containing 1,5g red P (0,05mol) they add 10mL bromine (0,20mol), and they distill bromoacetyl bromide.. No yields are
stated, so here thr red P might simply act as a source of PBR3..
|
I would guess that it's still PBr(x) formation in situ. Do take another look at your equation though. Keep in mind this is not a redox
reaction, so phosphorus is going to go from PBr3 to phosphorus acid. Phosphorus must pick up the "OH" from the carboxylic acid (I'll draw mechanism
if you want), and can do so three times. I.e. one mole phosphorus can yield three moles acyl bromide. So your rxn scheme sounds reasonable. Since
bromine is volatile it ought to be used in excess. Excess acetic acid, slight excess of bromine and phosporus is the limiting reagent. Sound
plausible?
EDIT: Okay I overlooked alpha bromination here. This does not require phosphorus (as evidenced by OrgSyn prep you referenced), but does require an
equivalent of bromine. So I would say the stoichiometry used is there is bit odd.
[Edited on 19-9-2009 by Arrhenius]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
The mechanism goes something like this:
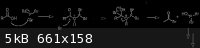
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Klute, this is the Hell-Volhard-Zelinsky halogenation of carboxylic acids. A small amount of PBr3 is formed and acts catalytically. The phosphorus
is often described as a halogen "carrier". See for example:
http://en.wikipedia.org/wiki/Hell-Volhard-Zelinsky_halogenat...
Better to remain silent and appear a fool than to open your mouth and remove all doubt.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Yes, I was aware of the formation of hypophosphorus acid, and it's reaction mecansim, including the a-halogenation of acids..
But the formation of acyl bromide from stoechiometric bromine and catalytic P (french ref)? How is the PBr3 regenerated? Or what is the
active species here?
EDIT: I'm going to try and be more clear: In the traditional a-halogenation of acids, a cat amount of P form PX3, which forms acyl halide, which can
enolize and be a-halogenated. This a-halogeneoacyl halide is more electrophilic than the acid (as described in the wiki article), so converts the acid
to a acyl halide, itseldf turning to a a-halogenoacid. The PBr3 just initates this catalytic cycle, it is consumed after one "round".
But the Ann. Phys. Chim. article describs the formation of acetyl bromide from acetic acid, Br2 and catalytic red P. So
something is constantly forming a brominating species capable of turning acetic acid to acetyl bromide, If it's still PBr3, it is regenerated
in some way..
2H3PO3 + 3Br2 --> 2PBr3 + 3HOBr? and HOBr --> HBr + 1/2O2?
That's the only explication I can find, although from a thermodynamic point of view this seems inplausible at the mild conditions this reaction is
performed.. This is what I don't understand!
So, following this idea, I postualted that adding a second equivalent of bromine will halogenate the acyl bromide to a-bromoacetyl bromide, which is
sdescribed in the two notes of the org syn prep.. Without forming the acid in-situ..
[Edited on 19-9-2009 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I don't think you can use catalytic phosphorus to access the acyl bromide. Atleast it would be better to use 1eq. You already pointed out that
there's no conservation of the oxygen atom (hydroxyl moiety). Think of thionyl chloride or oxalyl chloride. How do these create an acyl chloride? By
abstracting the oxygen from the carboxylic acid to give a gaseous side product, entropically driving the reaction.
It is possible that you could get HOBr or something like this, but I would not regard that as a desirable side product. But maybe it wouldn't matter
here. I can imagine that hypobromous acid would leave the reaction as a gas. But it doesn't seem realistic that it would arrise from what you've
drawn above. If so, everyone would be making PBr3 that way, don't you think?
[Edited on 19-9-2009 by Arrhenius]
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Klute, if you look at the "scheme" oj the Hell-Volhard-Zelinsky link from entropy51 it appears the P (actually the PBr3 it forms) acts more as an
initiator of a chain reaction rather than a traditional catalyst (being regenerated).- It is quite good explaination
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Yes, that's the whole point: as it initates the reaction, how can it form stoechiometric amounts of acetyl bromide in the french ref?
And I think that's why this could be interesting: forming acetyl bromide simply from Br2 and cat red P! That means Ac2O from acetic acid without any
thionyl chloride, phosphorus chlorides, sulfur, etc!! I mean, 1500G of AcBr from 750g AcOH using 2kg of Br2 is quite a good yield! Even though there
might be a little a-bromoacetyl bromide side product..
EDIT:
| Quote: | | I don't think you can use catalytic phosphorus to access the acyl bromide. Atleast it would be better to use 1eq. You already pointed out that there's
no conservation of the oxygen atom (hydroxyl moiety). |
I've just realized I haven't linked the french article, detailing the preparation of acetyl bromide from acetic acid, Br2 and catalytic red P.
http://gallica.bnf.fr/ark:/12148/bpt6k34857q.image.f81.langF...
Ann. Phys. Chim.; T17, p.83 (1879)
Here is a quite translation of the revelant part:
"Preparation of acetyl bromide-
2kg (~12,5mol) of bromine are added in small portions to 750g (~12,5mol) of GAA and 50g (1,61mol) of red phosphorus contained in a RBF equipped with a
efficient reflux condenser. The tube leading the bromine must be immersed under the surface of the liquid to avoid formation of crisatls of
bromoacetic acid that would otherwise plug the tube. Care must be taken to regualirily agitate the flask so that the reaction proceeds slowly. Once
the bromine is added, the mixture is refluxed for one hour, then a distillation setup is mounted and the product distilled on a hot water bath; 1500g
of acetyl bromide are thus collected.
We could have prepared the acetyl bromide by action of phosphorus bromide on glacial acetic acid, the yield even being possibly higher; but, apart
from the fact that anothe roperation is needed, the product must be distilled with great care, as it always contains notable quantities of phosphorus
bromide that produces dibromohydrine and epibromohydrine [they add the prepared acetyl bromide to glycerol to form acetobromohydrin]"
I doubt this procedure would be total bullshit, that was less common in thoses days 
Something worth trying IMHO.
[Edited on 20-9-2009 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Klute, I may be missing the point here, but I think you need catalytic amounts of PBr3 for the Hell-Volhard-Zelinsky, but molar amounts of PBr3 to
make AcBr. It wouldn't be the first time I was wrong.
Have you tried consulting an advanced organic chemistry text, such as Carey & Sundberg? I think some of these basic questions are better answered
in the textbooks than in ancient literature references. I have immense respect for the old literature, but sometimes it is less than accurate. Peer
review was not their strong point back then. Some of the reported reactions do occur but are very difficult to replicate with the reported yields.
Just a thought.
Maybe you should see if you can replicate the AcBr paper before putting too much faith in it?
And as always, I look forward to Nicodem clearing this up for us.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Well, my interest in this reaction is mainly for a-bromopropionyl bromide, considering I have acetyl chloride, acetic anhydride.
But I already have bought 50g of bromopropionyl bromide, so it's not something I'll be doing in any near futur, I'm just thinking of when I'll finish
thoses 50g I might be better off making it myself than buying it. Then again, I haev some a-bromopropionic acid and SOCl2, so I will surely make
a-bromopropionyl chloride before trying this out.. But I'm thinking of all those that don't have acces to such reagents, considering the number of
views for the acetic anhydride thread, I'm sure many will be interetsed in a preparation of acetyl bromide from Br2, GAA and cat red P..
Considering there are several mentions of the preparation of acetyl bromide by this method in at least two articles, including the one I posted above
"optimizing" the recation by using stoechiometric amounts of PBr3, I am failr confident this works.. But then again only a experiment can tell..
I too am looking forward to see Nicodem's views on this.. Hopefully he will be able to clear up the issu..
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|