| Pages:
1
2 |
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I now also obtained crystals from the yellowish solution. I put the solution in a warm place (around 50 C) for half a day and quite some crystals
collected. Nice fairly large crystals with a pink/brown color in daylight and a yellow/brown color in TL-light. These crystals are pink/rose in
tungsten light.
I assured that the liquid is not evaporated to dryness, approximately 1/4 of the liquid remained and this liquid had a deep yellow color. At that
point, I decanted the liquid, rinsed the crystals two times with boiling hot water, between rinses dapping them dry with a piece of filter paper.
After the final rinse the crystals were put on a filter paper to absorb the last remains of water and then they were dried (which only took one hour
or so, because they were almost dry).
A test for iron with concentrated thiocyanate shows negative results for the crystals. The same test, when applied to the remaining yellow liquid
shows a fairly strong red color, so the iron is concentrated in the liquid and not in the crystals.
Now I have two samples of neodymiumsulfate, a pink one and a brownish one. The brownish crystals dissolve in water very slowly, giving a
greyish/lavender solution, but not as nicely lavender as the solution, obtained from the pink solid.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
I have just ended recrystallization process of Nd sulfate.
Evaporating ~250 cm3 of H2O at ~40C was a little long lasting.
Effect - large crystals of Nd2(SO4)2x8H2O of light-violet colour with pink shade. Look like pieces of glass. These crystals possess high density -
this amount "looks like" ~10g but weights more than 20g.
Crystals of the sulfate are quite hard to crush and in powdered form are pink.
I will try to play with Nd2O3 and H2SO4 but I have to first prepare this oxide from oxalate (free from Fe).
*Picture of sulfate is attached (for the interested). Daylight, no flash lamp.
Attachment: dscn0079.rar (792kB)
This file has been downloaded 798 times
[Edited on 21-10-2009 by kmno4]
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Your crystal looks exactly like my crystals, I obtained from the yellow liquid. Yours is much bigger, but the color is exactly the same! My crystals
also are transparent like glass. The funny thing is, the crystals are from the yellow solution.
I also had another interesting observation. I took some Nd2O3 and dissolved this in excess amount of 65% HNO3 (some heating is required to get it all
dissolved). The resulting solution is yellow in TL-light and golden/brown in daylight! When I dilute this solution with around 5 times its volume of
water, its color immediately goes from yellow to lavender. The diluted solution is lvender, both in daylight and TL-light. So, in nitric acid such a
phenomenon also exists, but only in highly concentrated acid.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I would suggest the following
Make some more Nd2(SO4)3 as before, not too much excess H2SO4 or HNO3.
Add smakk amounts of Nd2O3, stir and wait for solution with each addition until it is clear that excess Nd2O3 has been added. Add water if needed to
keep the sulfate in solution.
Remove roughly 5% of the solution and precipitate the Nd as the hydroxide. Wash the ppt well, and the added it back to the main solution.
Add some H2O2 with stirring, then warm the mix. After a half hour separate the solids from the solution, then treat the solution to obtain Nd2(SO4)3
as before if you started with H2SO4, else just concentrate it a bit while observing colour.
This is a standard general procedure for obtaining higher purity salts from technical grade sources. It in particular removes Fe and Mn, but also can
remove Al and Si. Used to be used to purify technical CuSO4, which commonly contains FeSO4 that can not be removed by simple recrystallisation.
The thing you need to remember is the the REE have narrow absorption bands, in some cases very narrow while in other cases there is a cluster of
narrow bands that overlap as concentration is raised. On the other hand the transition metals' colours are generally from quite wide bands. The
result of this, besides the colour changes with varying light sources for REE but much less effect with the d-block metals, is that the d-block bands
can visually predominate at moderate concentration. The more intense lines of the REE become apparent with dilution as the d-block band fade out.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
My Nd salts are contaminated by something, beacuse Nd oxide preparated by oxalate Nd ignition has chocolate colour 
In some paper the same colour is ascribed to Pr contamination of Ln oxides. Redissolving this coloured oxide and precipitation oxalate again and then
ignition, does not change colour.
It is not Fe or Mn contamination , because precipitated hydroxide is white and does not change when H2O2 is added.
When the oxide is dissolved in HCl, weak smell of Cl2 appears[ possibly oxidation Cl(-) by Pr(IV)].
Presence of Pr also may explain light green colour of solution Nd salts in fluorescent light. I am dissapointed 
Probably magnet used for Nd extraction was not good quality.
My first try with small Nd magnet (from another source) gave nice blue-white Nd2O3.
Unfortunately I am not able to do UV-VIS absorption measurements, it would be the best way to check presence of Pr in my samples.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
What you observe indeed must be due to presence of Pr. My Nd2O3 (from eBay) is free of Pr, because I do not see the brown color on igniting salts
based on my Nd2O3 (Nd cannot go in oxidation state +4 by simply igniting the oxide in air). When I strongly heat precipitated Nd(OH)3 made with
ammonia, then I obtain a grey powder with a somewhat bluish/green hue. If I do the same after adding some PrCl3 before making the precipitate of
Nd(OH)3, then I also obtain a brown powder on strongly heating the oxide.
On a dutch chemistry forum there also is someone, who isolated Nd-salts from neodymium magnets and he had similar results. His oxide also had a dark
color. He had taken his magnets from old hard disk drives.
Saying that magnets with Pr in them are not good quality magnets of course is not correct. A magnet is not made for having high Nd-purity, it is made
to be a strong magnet and if that can be achieved with Nd which also has some Pr in it, then the magnet still is OK for what it is made for. For YOUR
purpose the latter is a bad quality magnet, but for most other's people purpose it probably is totally fine 
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
There is some old article* about "schwarzen Oxyde des Praseodyms", unfortunately in Übermenschen language.
As far I understand, PrO2 is not soluble in 5% acetic acid and Pr6O11 may be converted to Pr(3+) and PrO2. This procedure is also mentioned in
another article** and its authors make pure PrO2 from Pr6O11. After a few steps I should be able to separate Pr and Nd from Pr-Nd mixture. Currently
it is under my investigation. It looks simple on paper, I do not know if it works for mixture of oxides at all.
* DOI: 10.1002/zaac.19251490118
** DOI: 10.1021/ic50016a030
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
It is already some time ago that this discussion was active, but I finally found some time to make a webpage about the experiments and the preparation
of neodymium sulfate.
http://woelen.homescience.net/science/chem/exps/neodymium/in...
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
I decided to separate Pr-Nd mixture I have by classical methode:
crystallization of double nitrate, 3Mg(NO3)2x2(Nd,Pr)(NO3)3 x24H2O.
Starting solution was green coloured.
After many steps I got Pr-rich crystals, colourless with very weak green shade and Nd-rich crystals violet-pink coloured.
Pr-rich crystals give nice green colour in solution, but Nd-rich crystals give hard to describe colour - brown + yellow 
However this solution gives very nice, large amethyst-like violet crystals*. All these colours are given for fluorescent light.
Under tungsten light all solutions/crystals are more or less violet-pink.
I am going to convert this Mg/Nd nitrate to some Nd salt, possibly sulfate.
* foto:
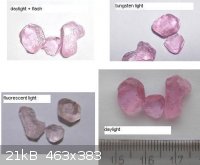
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kmno4  | It is interesting.
I also dissolved Nd2O3 in H2SO4 without strange colours.
My eyes noticed only the pinks violets...
Also known to me literature does not say anything about yellow/brown colours in the case of simple salts of Nd.
Even in case of solid Nd(HSO4)3 or (NH4)[Nd(SO4)2(H2O)3]H2O only light-violet colour is reported (Acta Cryst. (2006). E62, i169–i172 and
Z. anorg. allg. Chem. 624 (1998) 1583-1587).
Unfortunately the X-MET I have access does not see this metal and Ln at all (I think). It shows mixture Mn, Fe, Zn... etc instead of Nd. 
If anyone knows any literature about yellow or brown colored simple salts of Nd, it would be very desired to post it here. |
Here’s Wizzard with neodymium sulphate macro crystals obtained from neomagnets:
http://www.sciencemadness.org/talk/viewthread.php?tid=14145&...
The colour is unusual (but the material responds to TL/incandescent light in the usual way). No verification of any presence of iron has been made as
yet.
I’ve never precipitated or crystallised neodymium sulphate but I have obtained a K2SO4.Nd2(SO4)3.nH2O double salt, which despite being contaminated
with iron was light pink…
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Is PrO2 brown?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Brown to black, yes.
|
|
|
| Pages:
1
2 |