| Pages:
1
2 |
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
A Unique Synthesis of Ammonium Nitrate
In lieu of the general lack of ammonium nitrate availability in my area, I have devised a process which utilizes dollar-store items for the easy
manufacture of relatively clean ammonium nitrate. I thought I'd share it with the rest of the forum in the event that someone else was also in my
situation.
There are three main ingredients as well as a plethora of apparatus needed for this syntheses, all of which can probably be found around the home.
I start with household ammonia (the unscented kind, obviously), which is 5wt% ammonia. To this I add epsom salts, better known as magnesium sulfate,
to yield ammonium sulfate and insoluble magnesium hydroxide. After letting the magnesium hydroxide settle out, the solution of ammonium sulfate is
then boiled to concentrate it for the next step.
Cheap powdered drain unclogger contains three things: Sodium hydroxide, sodium nitrate, and aluminum turnings. As it turns out, it is incredibly easy
to sift the prills of calcium nitrate from the sodium hydroxide (which comes as much smaller prills). The calcium nitrate and aluminum mixture is
placed in just enough water to completely dissolve the nitrate, where it can be easily poured from the sunken aluminum turnings. This solution becomes
very cold upon the dissolution of the NaNO3, proving that there is minimal sodium hydroxide contamination present. The anti-caking agent also floats
to the surface and is easily removed in the decantation.
Finally, the sodium nitrate and the ammonium sulfate are combined and heated, yielding the white precipitate sodium sulfate and ammonium nitrate in
solution. This should be settled in the refrigerator as temperature has drastic effects on the solubility of sodium sulfate. The ammonium nitrate
solution is then decanted into a dish and desiccated for further use.
In all, I have found it possible to produce ammonium nitrate at a price comparable to purchasing cold packs - about $2 for 50 grams. Although more
complicated, it seems to have less impurities than the cheap, yellow-contaminated stuff in instant cold packs. Time to visit the dollar store - my
drains are clogged, my kitchen is dirty, and my feet could use a good soak. 
-DTM
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
And if your plants need feeding!
Get a real big bag of NH4NO3 fertiliser. . .
It is, I believe, very popular with farming folk!
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by hissingnoise  | And if your plants need feeding!
Get a real big bag of NH4NO3 fertiliser. . .
It is, I believe, very popular with farming folk! |
Would you please stop confusing the issue with facts!
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Quote: Originally posted by hissingnoise  | And if your plants need feeding!
Get a real big bag of NH4NO3 fertiliser. . .
It is, I believe, very popular with farming folk! |
Well I live on a farm and I am only a few Km from a fertiliser works but I can't buy ammonium nitrate (or potassium nitrate) there. I could a few
years ago, but not now. I can however buy CAN and easily remove the insoluble CaCO3. I not even sure pure ammonium nitrate is available here, even to
licensed applicators. You need to be a licensed applicator to purchase potassium nitrate. In Australia I believe even CAN is available only with a
license.
So just because you think ammonium nitrate is available to you (have you checked) it is not available to everyone.
Hissingnoise - you are rapidly becoming a pain in the arse post whore 
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
| Quote: | | Well I live on a farm and I am only a few Km from a fertiliser works but I can't buy ammonium nitrate (or potassium nitrate) there. I could a few
years ago, but not now. |
I wonder why NZ has determined that its citizens can't be trusted to buy
fertiilizer? Or maybe only certain citizens? Seems odd, doesn't it? I have to wonder why they have fertilizer works, but can't sell
the product. Seems like a loosing business propostion.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | | I have to wonder why they have fertilizer works, but can't sell the product. Seems like a loosing business propostion.
|
Do you actually know what fertiliser is!
There is more to fertiliser than just ammonium nitrate and potassium nitrate. Both of these are available in blends with trace elements and other
chemicals such as urea, ammonium sulphate, phosphates etc. but not as the "pure" material. It would be a pain in the arse to try and separate them
from the blended products. As far as a farmer is concerned, the blended products are far more useful anyway, albeit more expensive.
New Zealand along with Australia has decided it doesn't want it's citizens destroying buildings with trucks loaded with ammonium nitrate, or blowing
their fingers of with pipe bombs filled with home made gunpowder. Probably not an unwise choice given the present world situation.
|
|
|
ChrisWhewell
Hazard to Self
 
Posts: 66
Registered: 22-12-2009
Location: Austin
Member Is Offline
Mood: No Mood
|
|
Interesting theory. Not long ago, every farm town had a silo of NH4NO3 in the town, stocked with tons.
If one were part of a movement which wanted to disarm the populations of the West, then which threats would be top of that movement's list to remove
from the populace ??
My guess is that removing the nitrate silos would be on the top of the list, since a disarmed populace is unable to resist.
I think your stated position regarding people blowing their fingers off or demolishing buildings as an excuse to remove fertilizer silos is untenable,
given the long safe history of nitrate fertiliser usage over several decades and the lack of wrongful use of such materals is unsupported by the facts
of history.
It was a silent grab of hidden defences.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Huh! You must be joking! Does Oklahoma ring any bells.
I'm not sure what you guys are on about, you can still buy fertiliser in NZ. In fact this "clean and green" country probably has the highest per
capita use in the world. Just not in the "pure" nitrate form.
Anyway what does this have to do with some guy extracting ammonium nitrate from household items because he can't just buy them at the local
fertiliser works.
[Edited on 28-12-2009 by Xenoid]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Flattery! I love it!
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Doug, if you can get a large bag of CAN you'll have a good supply of cheap NH4NO3.
The insoluble binder is easily filtered out and what you're left with is reasonably clean NH4NO3.
If there are organic impurities colouring the nitrate heat it carefully close to its m.p..
This should give you a reatively pure, snow-white crystalline product.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
A couple of years ago in New Zealand, I bought at an auction for a very few dollars a container each of Ca(NO3)2 and of (NH4)2SO4, which were being
sold as soluble fertilizers. Quite a lot of it was being sold. One could easily dissolve these in water, mix them in stoichiometric quantities,
precipitate out the (only slightly soluble) CaSO4, and be left with a solution of NH4NO3 which could be crystallized out by evaporation (although
there may be a small amount of CaSO4 in it).
[Edited on 28-12-09 by JohnWW]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
John, if you needed 40 Kg. CAN, it should be fairly easy---even now!
http://www.ravensdown.co.nz/Products/Solid+Fertilisers/Nitro...
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hissingnoise  | Doug, if you can get a large bag of CAN you'll have a good supply of cheap NH4NO3.
The insoluble binder is easily filtered out and what you're left with is reasonably clean NH4NO3.
If there are organic impurities colouring the nitrate heat it carefully close to its m.p..
This should give you a reatively pure, snow-white crystalline product.
|
CAS 15245-12-2
http://www.agrium.com/products_services/ingredients_for_grow...
| Quote: | | Calcium Ammonium Nitrate (CAN 17)...The calcium in CAN 17 is water soluble and readily available to plants, |
http://www.chemicalregister.com/Calcium_Ammonium_Nitrate/Sup...
http://www.lookchemical.com/Nitric_acid_ammonium_calcium_sal...
https://irmm.jrc.ec.europa.eu/html/reference_materials_catal...
http://www.bluediamondtrades.com/docs/aus/CALCIUM_NITRATE_MS...
| Quote: | Synonyms: Nitric Acid, calcium salt, calcium nitrate,
CAS No: 15245-12-2
Molecular Weight: 1080
Chemical Formula: 5Ca(No3).NH4NO3.10H2O
|
No "insoluble binder", at least in those products with that CAS number. There may be some confusion because for fertilisers it is common to speak of
their calcium content in terms of the equivalent amount of limestone. From my experience it is not uncommon for Chinese CAN to have some true
limestone and sandy grit in it, which obviously would leave insoluble residue.
There is another grade of CAN that has NH4NO3 as the majority nitrate, with calcium nitrate added to make it effectively non-explosive; this has a
noticable higher nitrogen value. I used to see it in Canada, but that was years ago.
So you may need to research any CAN available, using the nitrogen (handy if they split out NH4 and NO3 values) and calcium content in order to
determine what you are getting and what proportions to mix.
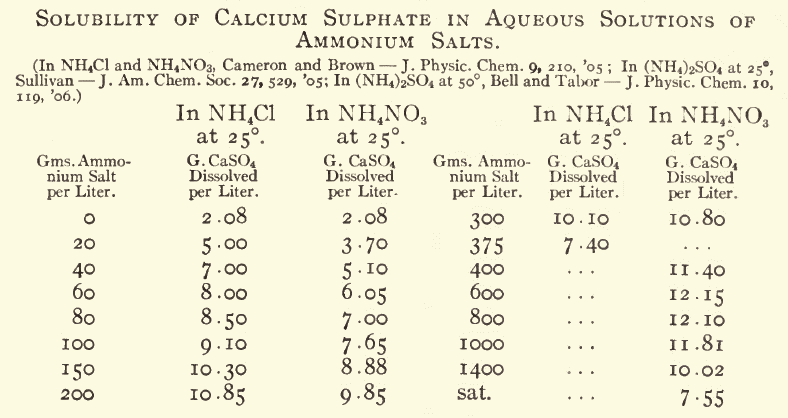
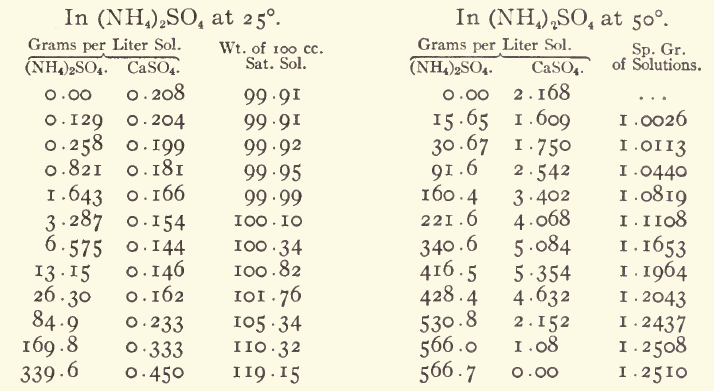
Also, as I have detailed many times before, calcium sulfate has its solubility increased by ammonium salts. I think the best way to make ammonium
nitrate from calcium nitrate and ammonium sulfate is to use a tiny bit less of the sulfate than needed, decant the solution away from the hydrated
calcium sulfate, add a solution of "ammonium carbonate" to the nitrate solution until CaCO3 ceases to precipitate, decant and filter to remove the
precipitate.
If you want really clean NH4NO3 then after crystallizing it from solution, dry it, mix in about 2% "ammonium carbonate", then dissolve it in hot
methanol, filter, and chill to crystallise. The MeOH can be used repeatedly to purify further batchs, avoiding most of the losses to the mother
liquor.
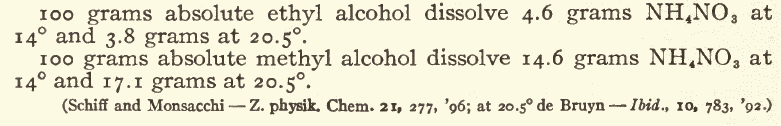
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
When you turn your attention to a subject n_i, it sure gets researched. . .
|
|
|
NBK=Hero
Harmless

Posts: 1
Registered: 3-5-2010
Member Is Offline
Mood: No Mood
|
|
They are always watching...kinda
My attempt to find and legally purchase AN resulted in a goon following me to my car in an attempt to get personal info from me so he could "call me
and let me know when that came in." I did not get in my car, nor did I let him know what car it was....I walked away and came back later.
FEDGOV has sent letters to these people demaning they keep track of people asking about these things.....i remember Mega warning against this line of
questioning for this exact reason. Beg, Borrow, Steal....or make your own.
|
|
|
gnitseretni
Hazard to Others
  
Posts: 280
Registered: 5-1-2007
Location: Medellin
Member Is Offline
Mood: No Mood
|
|
Haha.. great username.... NOT!!
|
|
|
stygian
Hazard to Others
  
Posts: 242
Registered: 19-9-2004
Member Is Offline
Mood: No Mood
|
|
I read only ~1/2 of this thread (got an Akromaster kit from my old man and I wanna go play with it!) but fwiw, I personally find the 'green movement'
quite interesting. A great many 'green reagents' seem to be quite useful in a good number of reactions, and with this 'green movement' going on,
people have found many simple synthesis of these reagents. As my primary or greatest fascination is in the industrial/raw materials sciences, I find
any synthesis involving these a very interesting read. Aromatics from cracking of vegetable fats, anyone?
|
|
|
simply RED
Hazard to Others
  
Posts: 206
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
Carbamide and mixed fertilizers are much more effective than ammonium nitrate to treat crops!
Disappearing of AN is not some kind of conspiracy, just economic consequence.
When logic and proportion have fallen sloppy dead...
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Quote: Originally posted by simply RED  | Carbamide and mixed fertilizers are much more effective than ammonium nitrate to treat crops!
Disappearing of AN is not some kind of conspiracy, just economic consequence. |
I beg to differ. The soil conditions & the specific plant are what makes one particular fertilizer more effective or less. There ARE some
conditions and plants that demand ammonium nitrate specifically as the base fertilizer / soil treatment for a specific seasonal overturn.
There was a senator from New York named Shumer who proposed simply BANNING all ammonium nitrate in a bill offer in the floor of the Senate. He was
shot down FAST as various agricultural states explained to him the facts of life. What has taken place is that no BAGGED ammonium nitrate is commonly
available. One may still buy it by the ton delivered in trucks via hoppers or swing shoots.
A very good illustration is Virginian & S. Carolina tobacco farming demand it due to acid soil conditions and the plant's very fast cycle. A mix
may not harm..... but it would cut profits since it would be more expensive than necessary, etc. There are some non-poisonous common grains also.
[Edited on 5-5-2010 by quicksilver]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | Virginian & S. Carolina tobacco farming demand it due to acid soil conditions and the plant's very fast cycle. |
I agree that AN will always be important as a fertiliser and I believe it's used to correct alkalinity in soils while supplying N.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
What about that failed would-be amateur Connecticut Pakistani Muslim car bomber in Times Square, New York, a couple of days ago, who was arrested on
board a plane about to take off for Dubai after the VIN number of the car was traced to him, and who has just appeared in Court? News releases from
the Pigs said a steel box full of "fertilizer" found inside the car bomb, and intended to provide the bomb's main explosive force, using ordinary
retail fireworks as a "detonator", was "the wrong type" of explosive, although without saying exactly what the stuff really was. The bomber, supposed
to be qualified with an MBA possibly from a diploma mill, obviously did not know the first thing about Chemistry; - he probably walked into a farm
supplies store, and grabbed a sack of something like (NH4)3PO4 or NH4MgPO4 ("AmmPhos") or (NH4)2SO4 instead of NH4NO3. He might have had a chance of
success if he had grabbed a sack of KNO3 or Ca(NO3)2 instead.
Even if the detonation of the fireworks had been sufficient to set off the bottles of propane and cans of gasoline which were in the car bomb, there
would not have been much of an explosion, but quite a large fireball which would have set the surroundings on fire.
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
By the way of manufacturing the AN is one of the most cheap N-fertilizers imaginable ... : A ton for 100 or 200 bucks ...
==> ... directly off the plant ...: Whenever HNO3 OR NH3 is in less demand it will be combined as AN-fertilizer, keeping an extra-market open ...
etc. ...
http://newsgroups.derkeiler.com/Archive/Rec/rec.gardens/2009...
| Quote: |
Fertilizer prices key on energy prices. With that in mind they should come down. Lawn fertilizer is basically nitrogen fertilizer. Manufacturing 1 ton
of anhydrous ammonia fertilizer requires 33,500 cubic feet of natural gas. When natural gas prices are $2.50 per thousand cubic feet, the natural gas
used to manufacture 1 ton of anhydrous ammonia fertilizer costs $83.75. If the price rises to $7.00 per thousand cubic feet of natural gas, the cost
of natural gas used in manufacturing that ton of anhydrous ammonia rises to $234.50, an increase to the manufacturer of $150.75. Most of the other
popular forms of nitrogen fertilizer are made with anhydrous ammonia. Urea is formulated by a reaction between anhydrous ammonia and carbon dioxide at
high temperature and pressure. Ammonium nitrate is formulated by combining anhydrous ammonia and nitric acid in a very corrosive manufacturing
climate. Solution liquid fertilizers (28 to 32 percent nitrogen) are composed of one-half urea and one-half ammonium nitrate. It's pretty hard to
apply a nitrogen fertilizer formulation that doesn't have natural gas in its manufacturing process. |
|
|
|
hiperion42
Hazard to Self
 
Posts: 75
Registered: 24-8-2009
Location: european mainland
Member Is Offline
Mood: overwhelmed
|
|
Quote: Originally posted by JohnWW  | | What about that failed would-be amateur Connecticut Pakistani Muslim car bomber in Times Square, New York, a couple of days ago...
|
People who started planning a major attack after 9/11 have had
almost 10 years which should be plenty for planning and making some kind of a big bomb.
If something would happen it should happen now give
or take a year. When nothing happens it will prove that
all the terrorist hysteria has ultimatly only served the policy of fear implemented by many governments.
I mean how the government officials managed to keep a straight face when they presented the
Homeland Security Advisory System is beyond me.
.....ejuu....................................................................Ffg..............................g.............
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
There was a senator from New York named Shumer who proposed simply BANNING all ammonium nitrate in a bill offer in the floor of the
Senate. He was shot down FAST as various agricultural states explained to him the facts of life. What has taken place is that no BAGGED ammonium
nitrate is commonly available. One may still buy it by the ton delivered in trucks via hoppers or swing shoots.
[Edited on 5-5-2010 by quicksilver] |
I didn't vote for him.
When I see the hopper full of AN sitting the yard of my neighbour
down the road .... I put my generator and shop-vac in the back
of my ATV. The day he puts in down.... I go out after dark and
vacuum some up.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Chicken shit
Quote: Originally posted by DougTheMapper  | In lieu of the
general lack of ammonium nitrate availability in my area, I have
devised a process which utilizes dollar-store items for the easy
manufacture of relatively clean ammonium nitrate. I thought I'd
share it with the rest of the forum in the event that someone else
was also in my situation. |
Jackson, Jr. & SM Kaye
Improvised Pyrotechnic Mixtures for Guerrilla Warfare Applications
Picatinny Arsenal 1964
A high order reaction from a black powder mixture w/ added
chicken-shit.
An infamous chemist suggested to me that CS contains A nitrate.
I do know for sure after standing down wind in a field parallel
to a field where the farmer was speading CS, while chuck hunting
— it contains a lot of ammonia and a really 'horid smell.
By da - What's the yellow stuff in chicken shit?
|
|
|
| Pages:
1
2 |