| Pages:
1
2 |
D4RR3N
Hazard to Others
  
Posts: 271
Registered: 9-1-2007
Member Is Offline
Mood: No Mood
|
|
Currently these air cars are using compressed air from a pump, I think they should use solid air which resembles dry ice. Go to the filling garage and
fill the tank with solid air pellets which would revert to compressed air at a controlled rate in a thermal vacuum sealed tank. This way filling time
would be like a regular car and mileage would be increased.
The only problem with hydrogen is a storage problem but I thing hydrogen from water using solar concentrator towers is the future. The oil fields of
the future may be replaced with fields of mirrors instead collecting sunlight and concentrating it at a central tower.
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
Ummmm... forgive me, but I'm laughing. I -really- want a process which would let me store oxygen in quantity. I work borosilicate glass in fuel/oxygen
torches. I use insane quantities of oxygen. I can get a very nice dewar with 200 liters of liquid oxygen. 7/8 or so of the bulk of it is insulation.
1/8 of it is oxygen. I have oxygen concentrators (8 of them) which can't keep up with my torch. If I could store oxygen while I was not working it
would be incredibly useful. The -best- option today to store oxygen from my concentrators is about $10,000 worth of pumps, $1000 worth of tanks, and a
-lot- of power. And I'd buy it if I could afford it, because in a few years it would probably pay for itself - I can easily use 8 tanks of oxygen in a
day. The alternative is a large garage-sized building containing an oxygen balloon - 5000 cubic feet or so would be really nice. The torch is about
30KW - in the range of a small car engine.
"solid air pellets" are somewhere around -230C. The amount of energy required to produce liquid air (or oxygen, nitrogen, etc.) is very large. Solid?
Much more.
Please do a little more research - there are very strong practical limits on insulation and cooling. If you work out a method for storing air or
equivalent gases at densities close to 1g/cm3 (even .1g/cm3) at room temperature, or a method for chilling to below -200C cheaply, and/or a method for
insulating a low temperature storage unit with really low heat leakage, you will be very very rich. None of these are easy problems. A lot of very
bright people have worked very hard on them. Strange chemistry and bizarre absorption might help you with storage. Thermodynamics puts hard limits on
chilling costs. Black body radiation, etc. put strong (though perhaps not insuperable) limits on insulation effectiveness. Aerogels are remarkably
good insulators but are not good enough for me to walk away from a tank of liquid oxygen for more than a few days without significant and expensive
losses.
Believe me, I'd be right in line for a storage unit like you propose! And every glass artist, scientific glassblower, and welding artist would be
right behind me!
Oh yes - current technology can produce batteries with very high charge and discharge rates. A car starter requires hundreds of amperes and those
batteries have been around for more than 75 years. Over 60 years ago submarines used banks of batteries for propulsion underwater (not easy to get air
for your diesels there!). So saying that heating, etc., make rapid charge BEVs impossible is false. Perhaps difficult? Yes. Not impossible. NiCd
batteries would be very good if they weren't so heavy and didn't contain truly nasty amounts of very expensive and poisonous cadmium. They have very
good discharge rates and good charge rates. NiMH batteries don't take high discharge rates well but are lighter and much less toxic. Li-ion batteries
are new (relatively) and may be ready in a few years - even a pessimistic extrapolation would give less than 10 years to a very high recharge rate.
A fuel cell which used hydrocarbons directly without a reformer and was otherwise cost effective would also be a ticket to a few billion dollars....
[Edited on 4-1-2010 by densest]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
What are you using 30kW of torch for? Could it be better served with a carbon arc torch or dielectric heating? (Hot glass absorbs microwaves pretty
good, maybe not such a great occupational hazard. High frequency induction ca. 13.56MHz may be useful.)
Tim
|
|
|
D4RR3N
Hazard to Others
  
Posts: 271
Registered: 9-1-2007
Member Is Offline
Mood: No Mood
|
|
I don't agree with you that exotic chemistry is needed to produce solid air pellets. The tank in the car would be like a large thermal tank
(reflective inner, vacuum sealed wall, thick insulation jacket).
The filling pump at the garage would only store a very small quantity of these pellets, more or less producing them as needed.
See video link and rough sketch
http://www.youtube.com/watch?v=grDe-iF_a6w
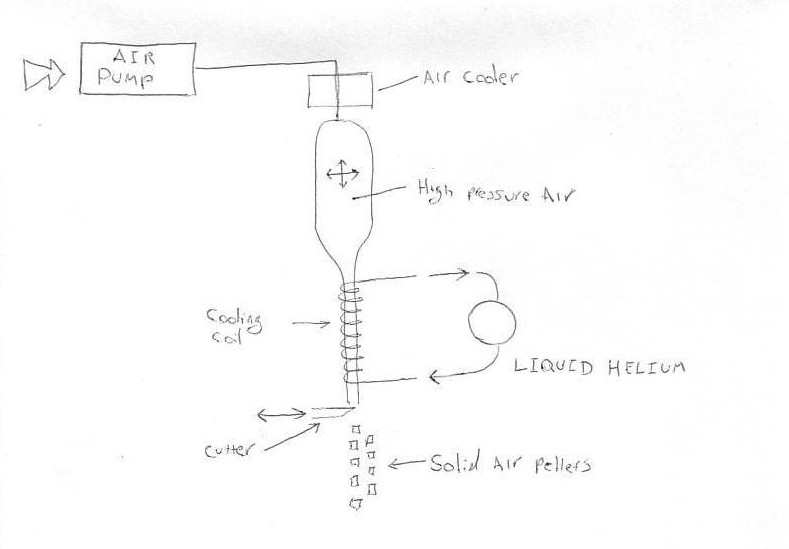
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | I don't agree with you that exotic chemistry is needed to produce solid air pellets. |
Well, that's one way to win an argument, at least in your own head: pretend that the other guy said something he didn't. It's the *energy costs*
associated with producing liquid air that pose the problem. Chemistry was suggested as something that might possibly help with storage.
The diagram you provide (besides suggesting a certain lack of discipline in learning to use new tools, as free CAD programs are easy to use) doesn't
tell us anything about how you would propose to deal with the end-to-end efficiency problems described above. Of course it's possible to produce solid
air via some series of steps corresponding roughly to your schematic. Now if you had actual engineering skills, you could look at each part of this,
spec out components that would do the job for some desired product throughput, and figure out roughly how much energy each of the machines used per
gram of air solidified. And then you could abandon your fantasy and move on to something more promising.
|
|
|
D4RR3N
Hazard to Others
  
Posts: 271
Registered: 9-1-2007
Member Is Offline
Mood: No Mood
|
|
bbartlog,
For your information I can use both autocad and solidworks, but I can do a sketch in 2 mins flat. Actually I happen to know quite a few Engineers and
product designers, you will find they all sketch too ;-)
I don't feel storage of the solid pellets is a massive problem either, yes they will gradually decompose into gas and the air pressure in your tank
will rise as this happens (you would have an air pressure release valve). You would also probably have to fill up every 2 or 3 days but would take no
more time then filling with regular fuels.
Efficiency, well with solid air pellets in the tank you would increase your mileage on a full tank. Efficiency of use to a end user may be more
important then the efficiency involved with the convention of compressed air to preform mechanical work.
Pumping oil out of the ground, refining it and then converting it to fuel suitable for use in an engine is hardly a very efficient process yet we do
it.
The energy to run the fuel system I sketched would mainly come from solar energy. Compressed air cars are already being produced, I just suggested
that their mileage could be increased and fill time reduced by using pellets instead of what they are using right now "compressed air".
http://www.youtube.com/watch?v=jgwfpIOOb-c&feature=relat...
[Edited on 4-1-2010 by D4RR3N]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
For transportation, efficiency is measured as Well-to-Wheel, the percentage of the source's energy that ends up turning the wheels of the vehicle.
The term 'well' means any energy source, for hydro the theoretical energy contentin the water behind the dam for example. For some sources, such as
wind where the limit on energy extraction is 59.3% (Betz limit), you could use either the total energy, the Betz limit energy, the mechanical power
actually captured, or the power out of the generator - but you should specify which you are using; generally in this case it is the electrical power
output of the generator. In some case it is important to specify the actual vs theoretical numbers, while wind turbines are generally specified by
their actual electrical output for a given wind speed, photovoltaic system should specify actual output and their conversion efficiency so that the
system's size can be estimated.
This can be broken down into Well-to-Pump, the losses in getting the energy from source to consumer, and Pump-to-Wheel, loses in the consumer's
utilisation of the stored energy.
So would you care to give numerical valuse for WtW, WtP, PtW for convensional petroleum fueled ICE, compressed air, your solid air idea, hyrogen
fueled vehicles - both ICE and fuel cell based, and Battery Electric Vehicles? Or are you just hand waving wth no real data?
|
|
|
Aurus
Harmless

Posts: 43
Registered: 31-12-2008
Member Is Offline
Mood: Oxidative
|
|
D4RR3N, it seems to me that Liquid Helium, which you propose using for cooling air is not exactly a household material... Not part of the usual
laboratory reagents either...
It is an expensive very low temperature liquid boiling at 4K.
Let those who seek, find, and those who find, seek ever more
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Quote: Originally posted by D4RR3N  | | Pumping oil out of the ground, refining it and then converting it to fuel suitable for use in an engine is hardly a very efficient process yet we do
it. |
Actually, it's on the order of ~500% efficient (i.e., a net producer of energy), while the methods you propose are on the order of 10% down to 0.1% or
less (highly wasteful).
Tim
|
|
|
bquirky
Hazard to Others
  
Posts: 316
Registered: 22-10-2008
Location: Perth Western Australia
Member Is Offline
Mood: No Mood
|
|
I think another major problem with air cars is a saftey issue. a presure vesle rupturing in an acident with that amount of energey in it is quite
posibly going to make you dissapear. sadly flywheels suffer from a simalar problem
at least with a petrol/LPG/CNG/syngas/whatever tank the speed of the reaction going to be limited by the avalible oxygen in a fire. i think electric
cars are potentialy very safe in this regard. and you can surley do some cleaver things in regards to positioning the mass of the batterys to benifit
crash saftey.
i dont think lithium cells are the answer though.. im not sure there is enough of it to go around 
what do the numbers look like for giant rubber bands ? 
|
|
|
| Pages:
1
2 |