overunity33
Harmless

Posts: 35
Registered: 3-1-2010
Member Is Offline
Mood: No Mood
|
|
Microwave safe, high temperature plastic?
I'm interested in making a microwave steam distillation setup for extracting essential oils from plant leaves. This would involve a container that
would be nuked for 30 minutes or so. I would love to use some type of large plastic tub, with a screw on lid for adding material. You guys got any
suggestions for something that will survive the microwaves and steam and stay inert?
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Wikipedia:
| Quote: |
For common commercial grades of medium- and high-density polyethylene the melting point is typically in the range 120 to 130 °C
|
So my shot would be HDPE.
Never tested this so i dont know if it will hold up in a microwave.
How about ceramics?
Certain oxides are very inert and have a very high temp range.
Btw
What kind of oils are you aiming at?
[Edited on 4-1-2010 by User]
What a fine day for chemistry this is.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Where are you planning to put the steam, now? Is this supposed to be a closed container, or are you trying to force the steam through your material?
I'm just trying to figure out here whether your plastic needs to resist high pressures as well as some heat.
Unless you have put a hole in your microwave to lead the steam out to a condenser, I think it would be hard to make it work. And regardless, I'd
recommend glass if you're going to do something in a microwave for 30 minutes. Even if the average temperature is within the limits for the plastic,
there are often hot spots.
Also I would be wary of the microwave, there seem to be quite a number of reactions that proceed at much lower apparent temperatures when driven by
microwave radiation. Which might include reactions that would make gunk of whatever it is you're trying to extract.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
HDPE and polypropylene won't take and sort of load or stress at 100 C. Polycarbonate is iffy.
Safe plastics for that temperature range include PTFE, polymethylpentene, polysulfone (PSU), polyethersulfone (PES) and polyphenysulfone (PPSU),
polyetheretherketone (PEEK), polyamide-imide (PAI), and various silicones.
The polysulfone type resins are often used in consumer goods that get hot - steamers and other cooking appliances.
Note that the oils your are after will likely diffuse into the plastic, and may extract additives from the plastic; essential oils tend to be decent
solvents.
Once again, it's tough to beat a good stainless steel or copper unit for this sort of application, when you're not using microwaves. For microwave
applications glass works, someone I know does that sort of thing using a resin/reaction kettle in a microwave tipped on one end to make the work area
taller than it is wide. They had to replace the standard metal clamps with some they fabbed out of glass fiber reinforced PEEK and wood bolts.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I would favor PTFE, if you wanted a high-temperature plastic, although it should preferably be a particularly high-molecular-weight or crosslinked
form. However, high-molecular-weight polycarbonate (particularly Lexan, obtained by reaction of bisphenol-A with COCl2 or CO2 under heat and pressure)
is supposed to be good up to 400ºC. Silicones may be able to withstand the temperature, but their mechanical rigidity is not good, being usually like
putty or rubber.
|
|
|
overunity33
Harmless

Posts: 35
Registered: 3-1-2010
Member Is Offline
Mood: No Mood
|
|
What about using a microwave safe material to clamp together 2 pyrex baking dishes with the mouths facing together. A silicone seal could be used
between the two and one of the dishes could be drilled to accept a glass tube exiting the microwave. Is the pyrex cookware the same grade as the lab
stuff?
Quote: Originally posted by bbartlog  | Where are you planning to put the steam, now? Is this supposed to be a closed container, or are you trying to force the steam through your material?
|
Its pretty much steam distillation, the microwaves boil the water in the cell walls, busting them and releasing the essential oils. The oils/water
travels through the material out a hole in the top of the container,then through a small tube at the top of the microwave which leads to your standard
coil condenser.
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
Absolutely not.Several threads on the shortcomings of what is calling itself "pyrex" cookware. UTSE.
It also sounds as though you're a little confused as to the techniques you plan to use.
"Microwave enhanced" extraction into various solvents including water is one thing.Co-distillation with water,whether the steam is generated in an
external boiler or in a resevoir beneath the suspended material is another.
Lots of threads on this,also books in the forum library,or general info on the net.
It may also be easier to look at solvent extraction and purification depending on your target.Certainly less involved than drilling,clamping bowls
etc(what were you planning on for clamps?) Than drilling through your oven etc.
If you care to share the class of compound you are trying to isolate,members here can be of more assistance.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
There are microwave pressure cookers made of a high strength, high temp plastic materal....just modify one of those?
|
|
|
overunity33
Harmless

Posts: 35
Registered: 3-1-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Eclectic  | There are microwave pressure cookers made of a high strength, high temp plastic materal....just modify one of those?
|
Dude, we have a winner! Beautiful in its simplicity. Thanks for everyone's advice I am off to track down a big microwave pressure cooker.
P.S. I attached a picture of what i am trying to make, replacing the round bottom flask with the pressure cooker
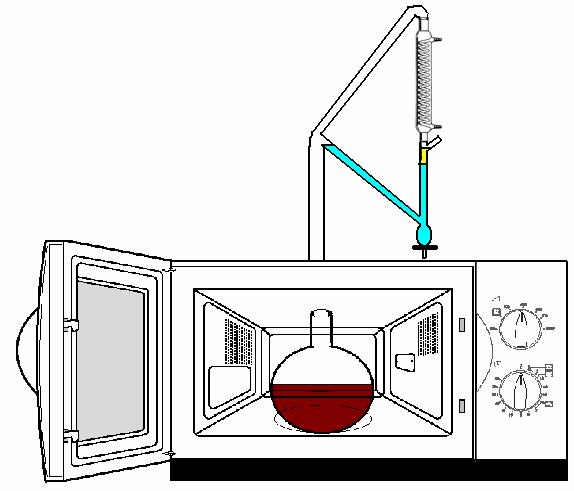
|
|
|
crazyboy
Hazard to Others
  
Posts: 436
Registered: 31-1-2008
Member Is Offline
Mood: Marginally insane
|
|
How do you plant to protect yourself from microwave radiation coming through that hole? Also why are you so convinced microwaves are special in the
ability to heat water inside cells? This isn't steam distillation as you aren't injecting steam into the boiling flask.
You would be better off using a standard steam distillation setup, regular old radiant heat can destroy cell walls just fine and you could use a
blender to chop up the plant material beforehand. If you are dead set on using a microwave to destroy the cells (I can't imagine why) just microwave
the plant materials beforehand then distill the oil.
This is a bad idea from a safety standpoint and a practical one.
|
|
|
overunity33
Harmless

Posts: 35
Registered: 3-1-2010
Member Is Offline
Mood: No Mood
|
|
A mesh cage might be built around the top of the microwave, either that or a large microwave absorbing metal choke around the glass tubing leaving the
microwave, mounted on the top of the microwave. I was under the impression that due to small diameter of the hole the 2.4ghz waves were not likely to
penetrate.
Article:
Microwave oven extraction of an essential oil
A. A. Craveiro 1, F. J. A. Matos 1, J. W. Alencar 1, M. M. Plumel 2
1Laboratorio de Produtos Naturals, Universidade Federal do Ceara, Caixa Postal 3010, Fortaleza, Ceara, Brazil
2Facullé de Pharmacie de Paris, France
Funded by:
FINEP
Keywords
Essential oil • Lippia sidoides Cham • Microwave oven • Steam distillation
Abstract
The essential oil, extracted by a current of air from the leaves of Lippia sidoides Cham, heated in a microwave oven for 5 minutes, was
qualitatively identical with that obtained by conventional steam distillation for 60-90 minutes.
Saves time really.
|
|
|