| Pages:
1
2 |
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
NH3, H2SO4, and HNO3 from fertilizer
Ammonium sulfate (NH4)2-SO4 is a common industrial by-product that's widely used as an acidifying nitrogen fertilizer. I can get it for $6 for a
20-pound bag. Heating it beyond its melting point yields NH3 and Ammonium bisulfate, which is quite acidic. Heating ammonium bisulfate causes an
equilibrium to be set up with NH3, H2O, and SO3 on one side and NH4HSO4 on the other side. Raising temperature and lowering pressure puts the
equation more in favor of the gases.
Now, platinum will catalyze the oxidation of ammonia to NO2. So what I'm thinking is, once the gases have started forming, I can start slowly pumping
in oxygen and run some current through a thin platinum wire I would have placed in the sealed flask prior to heating it. Once the wire gets hot
enough to catalyze the oxidation of ammonia, the exothermic reaction should keep it that hot for some time. However, I'm a bit worried that the NO2
would form nitric acid, react with the ammonia to form ammonium nitrate, then immediately decompose. Of course, that may be a minor side reaction
since NH3 would preferentially react with the sulfuric acid that would also be formed. Simultaneously distilling off the nitric acid somehow could
also drive the reaction in the preferential direction. Anyway, the end product would be a roughly equimolar mixture of concentrated nitric and
sulfuric acids, and we all know what we can do with that! 
Also, when making ETN, is there any danger in neutralizing the acid with NH4OH instead of baking soda? In that case, the solution could be evaporated
and the excess base would just evaporate too. The (NH4)2-SO4 would precipitate first, then the NH4NO3 could be dried later. This would have the
obvious advantage of providing a source of ammonium nitrate, I just hope there isn't also a chance of it blowing up on me. I don't think so because
it's in an aqueous solution, but that's what I thought about NI3, and I turned out to be quite wrong about that. 
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Catalytic from NH3 to NO2/x is a process that definitely needs controlled conditions.
If your planning to do this with acceptable yields in mind you should consider pressure/temp control and high a high efficiently absorption system.
Don't underestimate the reactions you are proposing.
I'd say show some nice reports.
[Edited on 26-2-2010 by User]
What a fine day for chemistry this is.
|
|
|
per.y.ohlin
Harmless

Posts: 27
Registered: 14-11-2009
Member Is Offline
Mood: No Mood
|
|
Ammonium nitrate is an extremely insensitive explosive. I don't know of any way to reliably detonate pure AN at room temperature, and ammonium nitrate
based explosives require a large booster to detonate. If you were to neutralize with ammonia, the ammonium nitrate would not be a significant
detonation hazard. I can't think of any possible side reactions when neutralizing with ammonia, so I believe it would be safe. As for a source of
ammonium nitrate, ice packs seem easier.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by per.y.ohlin  | | Ammonium nitrate is an extremely insensitive explosive. I don't know of any way to reliably detonate pure AN at room temperature, and ammonium nitrate
based explosives require a large booster to detonate. If you were to neutralize with ammonia, the ammonium nitrate would not be a significant
detonation hazard. I can't think of any possible side reactions when neutralizing with ammonia, so I believe it would be safe. As for a source of
ammonium nitrate, ice packs seem easier. |
Well, the only reason I was concerned was because the AN would be combined with ETN for a brief period, and there's always a chance they might not
separate completely. I agree, the chances that would cause problems are remote, but I just wanted to make sure. I was mostly interested in that
question because by neutralizing with ammonia, you can dump in as much as you want and not have to worry about rinsing it off later like you do with
baking soda, since it'll evaporate on its own. The fact that you get ammonium nitrate when you evaporate the liquid is a secondary benefit, but a
benefit nonetheless. 
Also, I spilled two beakers over because of all the bubbles from the baking soda, so I was looking for another base to use. I was going to use sodium
hydroxide, but didn't want to use something that strong in case it might damage the ETN. Another nice thing about neutralizing with ammonia is that
any nitric acid fumes are immediately visible in the vicinity when they combine with the ammonia vapor, so no more accidentally getting a whiff of
that stuff. The microscopic crystals that form do set off smoke detectors though.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
And how is this magic to be performed ? Most of this supposed process is
not detailed in the brief outline. If this is the extent to which it has been
thought out , it is not even a half baked idea.
The one pot preparation for nitric acid is a nitrate salt boiled in sulfuric acid.
Nitric acid made from the Oxidation of Ammonia is a multiple step process
not amenable to a single reaction procedure as suggested by this lightbulb
reactor. Ammonium Sulfate gives up the first Ammonia molecule at 100 ºC
Nitric acid boils at 120 °C before Ammonium Bisulfate melts at 147 ºC, and is
completely dissociated before further decomposition of Ammonium Sulfate
occurs at over 280 ºC. Oxidation of ammonia by a mantle of heated platinum
gauze occurs around 850 ºC. The most important issue is not even addressed ,
how is the necessary oxygen to be supplied.
Nitric acid is made from the oxidation of Ammonia in three stages. The first
step is the oxidation of ammonia gas ( NH3 ) with air to form nitric oxide ( NO )
by passing through a screen of platinum-rhodium catalyst at high temperature
and pressure to achieve a high conversion efficiency. The nitric oxide is cooled
and further oxidized with more air to form nitrogen dioxide ( NO2 ). This is
absorbed in water forming nitric acid ( HNO3 ) and nitrous acid ( HONO ) which
itself is further oxidized to nitric acid. Temperature and conditons at each step
require the contents to be segregated in their own separate reaction chamber.
This is described in greater detail below along with references and a process
patent attached.
. . . 4 (NH4)2SO4 => 4 NH4HSO4 + 4 NH3
Ammonia oxidation
1 ) . 4 NH3 + 5 O2 => 6 H2O + 4 NO
Nitric Oxide oxidation
2 ) . .4 NO + 2 O2 => 4 NO2
Nitrogen Dioxide absorption
3 ) 4 NO2 + 2 H2O => 2 HNO3 + 2 HONO
. . . 2 HONO + O2 => 2 HNO3
The overall reaction is _ 4 NH3 + 8 O2 => 4 H2O + 4 HNO3
Twice the molar volume of oxygen to that of ammonia is needed.
The equal molar volume of water also produced without a means of drying
will limit concentration so it cannot exceed ~ 75 % at best.
_________________________________________________
References
http://www.backyardchem.com/nitric-acid.php
http://chemistry.slss.ie/resources/downloads/ch_cw_nitricand...
http://www.platinummetalsreview.com/pdf/pmr-v11-i1-002-009.p...
Production Process Nitric acid is commercially available in two forms: weak (50 to 70
percent nitric acid) and concentrated (greater than 95 percent nitric acid). Different
processes are required to produce these two forms of acid. For its many uses, weak
nitric acid is produced in far greater quantities than is the concentrated form. Virtually
all commercial production of weak nitric acid in the United States utilizes three common
steps: (1) catalytic oxidation of ammonia ( NH3 ) to nitric oxide ( NO ), (2) oxidation of
nitric oxide with air to nitrogen dioxide ( NO2 ), and (3) absorption of nitrogen dioxide in
water to produce "weak" nitric acid.
The first step of the acid production process involves oxidizing anhydrous ammonia over
a platinum-rhodium gauze catalyst to produce nitric oxide and water. The exothermic
reaction occurs as follows: 4 NH3 + 5 O2 -> 4 NO + 6 H2O + heat. This extremely rapid
reaction proceeds almost to completion, evolving 906 kilojoules per mole ( kJ/mole )
(859 British thermal units per mole [Btu/mole]) of heat. Typical ammonia conversion
efficiency ranges from 93 to 98 percent with good reactor design. Air is compressed,
filtered, and preheated by passing through a heat exchanger. The air is mixed with
vaporized anhydrous ammonia and passed to the converter. Since the explosive limit of
ammonia is approached at concentrations greater than 12 mole percent, plant operation
is normally maintained at 9.5 to 10.5 mole percent. In the converter, the ammonia-air
mixture is catalytically converted to nitric oxide and excess air. The most common
catalyst consists of 90 percent platinum and 10 percent rhodium gauze constructed
from squares of fine wire. Up to 5 percent palladium is used to reduce costs.
Operating temperature and pressure in the converter have been shown to have an
influence on ammonia conversion efficiency. Generally, reaction efficiency increases
with gauze 8 temperature. Oxidation temperatures typically range from 750 ºC to 900 ºC .
Higher catalyst temperatures increase reaction selectivity toward NO production, while
lower catalyst temperatures are more selective toward less useful nitrogen ( N2 ) and
nitrous oxide (N2O). The high-temperature advantage is offset by the increased loss
of the precious metal catalyst. Industrial experience has demonstrated and the industry
has generally accepted conversion efficiency values of 98 percent for atmospheric
pressure plants at 850 ºC and 96 percent for plants operating at 0.8 megaPascals
(MPa) (8 atmospheres [atm]) and 900 ºC. As mentioned earlier, the ammonia oxidation
reaction is highly exothermic. In a well-designed plant, the heat byproduct is usually
recovered and utilized for steam generation in a waste heat boiler. The steam can be
used for liquid ammonia evaporation and air preheat in addition to nonprocess plant
requirements. As higher temperatures are used, it becomes necessary to capture
platinum lost from the catalyst. About 300 milligrams per ton of nitric acid is lost this
way. Consequently, a platinum recovery unit is frequently installed on the cold side of
the waste heat boiler. The recovery unit, composed of ceramic-fiber filters, is capable
of capturing 50 to 75 percent of the platinum lost by Nitric Oxide oxidation.
The nitric oxide formed during the ammonia oxidation process is cooled in the cooler /
condenser apparatus, where it reacts noncatalytically with oxygen to form nitrogen
dioxide and its liquid dimer, dinitrogen tetroxide. The exothermic reaction, evolving 113
kJ/mole 4 (107 Btu/mole), proceeds as follows: 2 NO + O2 -> 2 NO2 + heat. This slow,
homogeneous reaction is highly temperature- and pressure-dependent. Lower
temperatures, below 38 ºC, and higher pressures, up to 800 kilopascals (kPa) (8 atm),
ensure maximum production of NO2 and minimum reaction time. Furthermore, lower
temperatures and higher pressures shift the reaction to the production of NO2 ,
preventing the reverse reaction ( dissociation to 2 NO and O2 ) from occurring.
The final step for producing weak nitric acid involves the absorption of NO2 and N2O4
in water to form nitric acid ( as NO2 is absorbed, it releases gaseous NO ). The rate
of this reaction is controlled by three steps: (1) the oxidation of nitrogen oxide to NO2
in the gas phase, (2) the physical diffusion of the reacting oxides from the gas phase
to the liquid phase, and (3) the chemical reaction in the liquid phase. The exothermic
reaction, evolving 135 kJ/mole (128 Btu/mole), proceeds as follows: 3 NO2 (g) + H2O
-> 2 HNO3 (aq) + NO(g) + heat , and also 3 N2O4 (liq) + 2 H2O => 4 HNO3 + 2 NO(g)
The absorption process takes place in a stainless steel tower containing numerous
layers of either bubble cap or sieve trays. The number of trays varies according to
pressure, acid strength, gas composition, and operating temperature. Nitrogen dioxide
gas from the cooler/condenser effluent is introduced at the bottom of the absorption
tower, while the liquid dinitrogen tetroxide enters at a point higher up the tower.
Deionized process water is added at the top, and the gas flows countercurrent to both
liquids. Oxidation occurs in the free space between the trays, while absorption takes
place in the trays. Because of the high order of the oxidation process in absorbers,
roughly one-half the volume of the absorber is required to absorb the final 3 percent
of nitrogen oxide gas concentration. Because lower temperatures are favorable for
maximum absorption, cooling coils are placed in the trays. Nitric acid in concentrations
of 55 to 65 percent is withdrawn at the bottom of the tower. Secondary air is used to
improve oxidation in the absorption tower and to bleach remaining nitrogen oxides from
the product acid. Absorption efficiency is further increased by utilizing high operating
pressure in the absorption process. High-pressure absorption improves efficiency and
increases the overall absorption rate. Absorber tail gas is reheated using recovered
process heat and expanded through a power recovery turbine. In a well designed plant,
the exhaust gas turbine can supply all the power needed for air compression with
excess steam available for export.
.
Attachment: Manuf of Nitric Acid from Ammonia US1872638.pdf (462kB)
This file has been downloaded 940 times
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | The one pot preparation for nitric acid is a nitrate salt boiled in sulfuric acid. |
That's it in a nutshell!
For the amateur there's really no other practical route to strong HNO3.
Use K or NaNO3 - the ammonium salt produces a diluted HNO3 because the NH4 ion is oxidised by hot H2SO4.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
| Quote: | [quote=172624&tid=13434&author=franklyn]
And how is this magic to be performed ? Most of this supposed process is
not detailed in the brief outline. If this is the extent to which it has been
thought out , it is not even a half baked idea. |
The reaction can be carried out in ways other than exactly how nitric acid plants do it, you know. See:
http://www.chem.umn.edu/services/lecturedemo/info/catalysis_...
| Quote: | | The one pot preparation for nitric acid is a nitrate salt boiled in sulfuric acid. |
Yeah, I know. I've done that reaction already.
| Quote: | Nitric acid made from the Oxidation of Ammonia is a multiple step process
not amenable to a single reaction procedure as suggested by this lightbulb
reactor. |
I just have to generate the NO. The rest takes care of itself, provided O2 and water are present.
| Quote: | Ammonium Sulfate gives up the first Ammonia molecule at 100 ºC
Nitric acid boils at 120 °C before Ammonium Bisulfate melts at 147 ºC, and is
completely dissociated before further decomposition of Ammonium Sulfate
occurs at over 280 ºC. Oxidation of ammonia by a mantle of heated platinum
gauze occurs around 850 ºC. |
Well, your HNO3 boiling point is wrong, you're thinking of the water azeotrope which doesn't apply thanks to the sulfuric acid present. Without water
the boiling point is 83ºC.
Also, when ammonium bisulfate decomposes, did you ever think about what it decomposes into? Well, turns out it decomposes into SO3, NH3, and H2O.
SO3, as we all know, reacts with water to make sulfuric acid, and NH3 reacts with sulfuric acid to make ammonium bisulfate.
| Quote: | The most important issue is not even addressed ,
how is the necessary oxygen to be supplied. |
How is that the most important issue? Oxygen is readily available in the form of air, oxygen tanks, or chlorine bleach mixed with hydrogen peroxide.
I think it's a lot more important to make sure the NH3 doesn't become N2, at least for the most part.
| Quote: | Nitric acid is made from the oxidation of Ammonia in three stages. The first
step is the oxidation of ammonia gas ( NH3 ) with air to form nitric oxide ( NO )
by passing through a screen of platinum-rhodium catalyst at high temperature
and pressure to achieve a high conversion efficiency. The nitric oxide is cooled
and further oxidized with more air to form nitrogen dioxide ( NO2 ). This is
absorbed in water forming nitric acid ( HNO3 ) and nitrous acid ( HONO ) which
itself is further oxidized to nitric acid. Temperature and conditons at each step
require the contents to be segregated in their own separate reaction chamber.
This is described in greater detail below along with references and a process
patent attached.
. . . 4 (NH4)2SO4 => 4 NH4HSO4 + 4 NH3
Ammonia oxidation
1 ) . 4 NH3 + 5 O2 => 6 H2O + 4 NO
Nitric Oxide oxidation
2 ) . .4 NO + 2 O2 => 4 NO2
Nitrogen Dioxide absorption
3 ) 4 NO2 + 2 H2O => 2 HNO3 + 2 HONO
. . . 2 HONO + O2 => 2 HNO3
The overall reaction is _ 4 NH3 + 8 O2 => 4 H2O + 4 HNO3
Twice the molar volume of oxygen to that of ammonia is needed.
The equal molar volume of water also produced without a means of drying
will limit concentration so it cannot exceed ~ 75 % at best. |
Did you catch the part about simultaneously producing H2SO4? That's a most excellent drying agent, you know. And even though the industrial process
is complicated, it's that way mainly for three reasons: to maximize efficiency, provide a continuous process, and avoid releasing nitrogen oxides into
the environment. Efficiency is not that critical to me, I'm not producing enough to generate significant pollution, and I don't care about having a
continuous process, so no need for all those complicated optimizations.
[Edited on 2/26/10 by Melgar]
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
I absolutely adore your optimism.
| Quote: |
And how is this magic to be performed ? Most of this supposed process is
not detailed in the brief outline. If this is the extent to which it has been
thought out , it is not even a half baked idea.
The reaction can be carried out in ways other than exactly how nitric acid plants do it, you know. See:
http://www.chem.umn.edu/services/lecturedemo/info/catalysis_...
|
This is a demo that is well known, it does not produce any significant amounts for sure.
[Edited on 26-2-2010 by User]
What a fine day for chemistry this is.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by hissingnoise  | | Quote: | | The one pot preparation for nitric acid is a nitrate salt boiled in sulfuric acid. |
That's it in a nutshell!
For the amateur there's really no other practical route to strong HNO3.
Use K or NaNO3 - the ammonium salt produces a diluted HNO3 because the NH4 ion is oxidised by hot H2SO4.
|
Not quite. Ammonium nitrate decomposes at high temperatures, sulfuric acid or not. The NO3 ion is what oxidizes the NH4, which sucks because it also
lowers your yields. Thanks for the advice though, I'm just throwing out an idea for potentially cheaper materials since both sulfuric acid and
nitrate salts have been fairly expensive and/or hard to find lately. Considering I can buy a 20-pound bag of ammonium sulfate for less than the price
of a liter of sulfuric acid, I think it could be a viable process.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: |
Not quite. Ammonium nitrate decomposes at high temperatures, sulfuric acid or not. The NO3 ion is what oxidizes the NH4, which sucks because it also
lowers your yields. |
Just to be clear, NH4NO3 begins to decompose above ~174*C but when in excess, H2SO4 decomposes NH4NO3 above ~ 100*C.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by hissingnoise  | | Quote: |
Not quite. Ammonium nitrate decomposes at high temperatures, sulfuric acid or not. The NO3 ion is what oxidizes the NH4, which sucks because it also
lowers your yields. |
Just to be clear, NH4NO3 begins to decompose above ~174*C but when in excess, H2SO4 decomposes NH4NO3 above ~ 100*C.
|
In other words H2SO4 catalyzes the decomposition of NH4NO3 at lower-than-usual temperatures? That's what I'd expect given H2SO4's propensity to push
reactions in whatever direction favors the formation of water.
| Quote: | | This is a demo that is well known, it does not produce any significant amounts for sure. |
Well, considering the platinum is releasing enough energy to stay red hot, and considering the reaction on platinum favors the production of NO, then
either a significant amount of NO is being produced or that's a perpetual energy machine there. Either way it's a winner. 
[Edited on 2/26/10 by Melgar]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
NH4NO3 is still sold in 15.5-0-0 as "calcium nitrate". Why attempt such a thing if the material is freely available?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Whether hot H2SO4 is acting as oxidiser or catalyst is a moot point in this case.
But if you have difficulty getting H2SO4 or Na/KNO3 then you have a real problem because the other "method" you've outlined will mean a hell of a lot
of time and effort for very little product.
And prices for KNO3-H2SO4 are unlikely to go beyond anyone's means. . .
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
| Quote: | Whether hot H2SO4 is acting as oxidiser or catalyst is a moot point in this case.
But if you have difficulty getting H2SO4 or Na/KNO3 then you have a real problem because the other "method" you've outlined will mean a hell of a lot
of time and effort for very little product.
And prices for KNO3-H2SO4 are unlikely to go beyond anyone's means. . . |
Honestly, it's largely just to see if I can do it, and what the yields are like if I can. Plus, if it goes really well then it wouldn't be much more
work than making HNO3 from H2SO4 and KNO3, only instead of using H2SO4 I'd be getting it, as well as ammonia gas. But all in all, I think it's a cool
exercise in theoretical chemistry, and I already have all the stuff except for a platinum/rhodium thermocouple wire that cost me $30. We'll see how
it goes! 
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Blind optimism - that's the spirit!
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
There was an old patent here around after which NH4NO3 was the _preferred_ nitrate for heating with H2SO4, since everything melts nicely and the
reaction is more complete thereby ... ; also the paper said the HNO3 were quite clean from Water ...
The H2SO4 would come from electrolysis of a sulfate ...
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
My experience was that RFNA came over at the start but this gradually lightened as distillation progressed and near the end the distillate consisted
of water, essentially.
I don't remember (I may have) if I used an excess of H2SO4.
An NH4NO3/H2SO4 mix would be preferred for nitration of say, erythritol but I won't use it for HNO3. . . | Quote: | | The H2SO4 would come from electrolysis of a sulfate ... |
That electrolysis needs an inert anode like Pt!
Can't you just use drain-cleaner?
[Edited on 26-2-2010 by hissingnoise]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ Melgar
You have not yet given a lucid description of this imagined process you refer to
apart from randomly citing unrelated reactions in no coherent sequence without
stipulating reaction constraints.
If heated in confinement , Ammonium Sulfate dissociates as a high pressure
supercritical gas of steam , Sulfur trioxide and Ammonia , which may dissociate into
nitrogen and hydrogen itself , reducing Sulfur trioxide into Sulfur dioxide and steam.
How to enter air / oxygen into this bomb and vent the vessel remains unexplained
a need apparently not understood by you. What becomes of the supposed ( NOx )
formed as it accumulates at high temperature and pressure since it surely must
decompose. But it gets worse , the catalytic oxidation process is necessarily anhydrous
and the Sulfur oxides are not going to just stand by and only observe. Feeding this
stew into a platinum wire mesh is not going to yield nitric oxide ( NO ) in any amount
that is not hot off the catalyst , since the tendency will be to form water and free
nitrogen. That will certainly put a stop to the pressure buildup - in an instant.
Ignoring that temperature and pressure are all that matters in thermal equilibrium ,
in just the precisely needed optimal amounts at each separate stage , is not a plan.
(NH4)2SO4 => 2 H2O + SO2 + N2 + 2 H2 , <- this is the likely result.
Imperial Chemical Industries in England , developed a high temperature process for
conversion of Ammonium bisulfate to produce Ammonia and Sulfur trioxide. Ammonia
is further oxidized under these oxidative conditions to nitrogen and water , which is
added to the Sulfur trioxide producing Sulfuric acid , the end product.
a similar process patent US20080056983 is covered here , the prior art and research
cited is what is important , a sampling is below _
http://www.freshpatents.com/Process-for-producing-ammonia-an...
U.S. Pat. No. 4,081,515 (Gruhier) carries out the decomposition of ammonium bisulfate
by heating it to 400 ºC. The heating is carried out without a catalyst present or with
copper, molybdenum, or tungsten catalyst present. The primary product is sulfur dioxide,
which is not desired in the recovery of ammonia and sulfuric acid.
U.S. Pat. No. 3,282,646 (Bonfield) discloses the production of ammonia and sulfur
dioxide from ammonium sulfate. The ammonium sulfate was heated to 250 ºC. to
drive off ammonia and produce ammonium bisulfate. The temperature was increased
to 450 ºC. and carbon monoxide, hydrogen sulfide, hydrogen, or nitrogen was bubbled
through the molten salt. The reaction produced ammonia and sulfur dioxide, the latter
being an undesirable byproduct.
Halstead (J. Appl. Chem., 1970, 20:129-132) describes the decomposition of ammonium
sulfate at 400 ºC. The first reaction converts ammonium sulfate to ammonium bisulfate
and ammonia. In a second reaction, the ammonium bisulfate dehydrates to form a water
molecule and ammonium pyrosulfate, (NH4)2S2O7. Further heating of the ammonium
pyrosulfate to form sulfur dioxide and nitrogen, which are not desired products from
ammonium sulfate, is also carried out.
Kiyoura and Urano (Ind. Eng. Chem. Process Des. Develop., 1970, 9(4):489-494) describes
the thermal decomposition of ammonium sulfate to ammonia, sulfur dioxide, sulfur trioxide
and other gases. Kiyoura states that the simple decomposition of ammonium bisulfate to
sulfuric acid and ammonia is not an adequate mechanism to describe the thermal
decomposition reaction.
Liske et al. (Journal of Hazardous Substance Research, 2000, 2:8.1-8.17) teach the
complete thermal decomposition of ammonium sulfate to several gaseous products.
This combustion process does not recover ammonia and sulfuric acid.
Dugger et al. (Ammonium Sulfate Decomposition, 1955, RMO-2036, United States
Atomic Energy Commission) describes the thermal decomposition of ammonium sulfate
in the presence of zinc oxide. The reaction products are ammonia and sulfur dioxide.
.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Thanks for all the patent links, even though the tone of your responses indicates you aren't really trying to be helpful. At sub-plasma temperatures,
I doubt I have to worry about a significant amount of ammonia disassociating into N2 and H2, though I probably do have to worry about NH3 + O2 = N2 +
H2O.
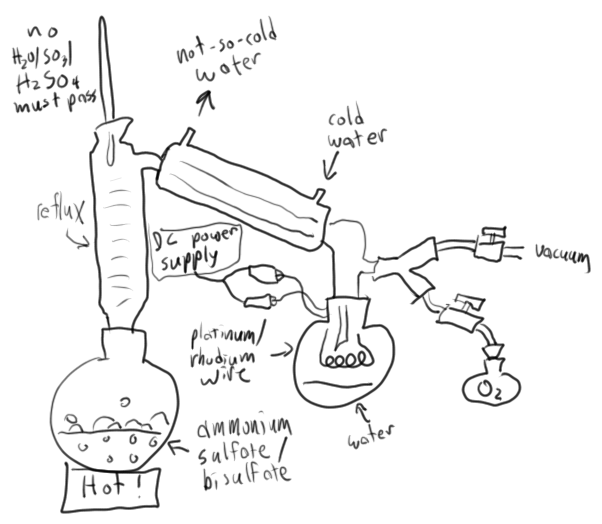
I drew a picture of my proposed setup, which is basically just a modified vacuum distillation set, not a bomb. I admit I wasn't sure what it should
look like at first, which is why I was soliciting advice, but I think I have an idea now. The vacuum distillation setup will be run in a way such
that no liquids are carried over to the collection flask, just gases. And the water would collect the nitrates that form, probably in the form of
nitric acid and ammonium nitrate. Potassium hydroxide could go in there too if someone wanted to make KNO3.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
What can one say - it's certainly artistic. . .
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
@Melgar :
your O2 and vacuum may need some alteration or you'll just loose the O2 to no avail.
@ hissingnoise :
I've not seen a flask set up for electrodes. IS there such a commercial thing? I thought about a 3 neck and a stopper but the heat may screw even a
polypropylene stopper up in short order. I suppose you could use a tweaked Titrator but that's serious money. I suppose if you kept the whole thing on
a really small scale you could get away with a stopper....
[Edited on 28-2-2010 by quicksilver]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | . I suppose if you kept the whole thing on a really small scale you could get away with a stopper.... |
The wire would have to sealed into the stopper to be dependable, and that would call for glassworking skills.
And dependable, in this case just means unlikely to leak.
For one thing, with the temperatures needed for the reaction, cold water in the condenser could be problematic. . .
Also, I'd say franklyn has it just about right!
[Edited on 28-2-2010 by hissingnoise]
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
| Quote: |
The wire would have to sealed into the stopper to be dependable, and that would call for glassworking skills.
And dependable, in this case just means unlikely to leak. |
I've successfully made mostly-tight seals for wires to flasks two ways, as long as the pressure differential isn't more than .1 bar or so. One is to
grind grooves down the taper of a glass standard taper stopper, then run the wires in the grooves, wrapping them in plumber's teflon tape. The other
way is to carefully put wires inside teflon tubing and heat the assembly until the teflon softens but does not decompose - it won't
really melt. Fill out to a suitable size with larger diameter teflon tubing or tape. Compress each wire into a hole in a teflon stopper with a hollow
tool until the plastic firmly solidifies. A zig-zag or partial loop in the wire helps prevent the wire from sliding which would enlarge the hole.
Neither method will make a gastight seal since teflon will not adhere to the metal. It is sufficient to keep liquid inside if not pressurized or at
depth, mostly.
I will assert that I think the proposed method would not have a good yield. If the (NH4)2SO4 is really really cheap or HNO3 is really unavailable, it
might work. I wonder about miscellaneous reactions between NH3 and SOx where NH3 reduces the SO3 to SO2? Perhaps some V2O5 heated in the gas + oxygen
stream might help? Or would it just confuse things?
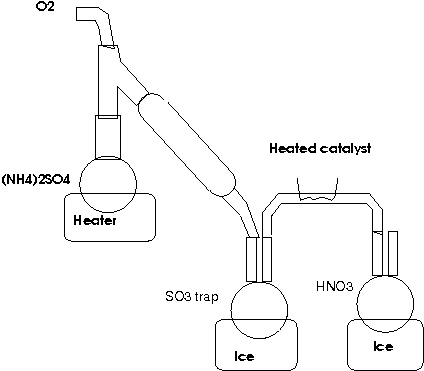
One might try to separate the SO3 from the NH3:
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
No such drain-cleaner in germany ... ; anyhow Sulfates are everywhere, and cheaply ...
==> Carbon rods for welding should do it, or not ? After all they even stand the far more agressive chlorate-electrolyses-environment for a while
...
For distillation of HNO3 from Nitrate it also would be sufficient to boil down a H2SO4-solution with residual sulfate, as it doesn't harm the
HNO3-generation ...
So the input for the process would be some sulfate-fertilizer and NH4NO3 ... ; only: What about the electrolysis of ammonium-sulfates ?
==> Might that become dangerous (reminding of the NH4Cl-electrolytical dangers) ?
==> Then the NH4NO3 would have to be converted with soda to NaNO3 + NH3 ...
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Hmm, thanks for the input.
Quicksilver - Obviously I'd only have one valve open at a time. The vacuum valve would probably only be open for a short time at the beginning, and
maybe later to get rid of inert gases if they're building up.
Densest - you have a good point with the possible SO3 + NH3 = SO2 + N2 + H2O reaction. However, I am hoping that SO3 reacts preferentially with H2O
and condenses out fairly quickly. I'm also counting on the fact that of the decomposition products, only NH3 is a gas at room temperature. I think
I'll also rearrange my distillation setup so that the condensation in the condenser runs back to the reflux column. As for the wires, they are very
thin; .005 inch diameter. They can run right through the silicone grease at any joint. And I only need to heat them once at the beginning. The
oxidation reaction is quite exothermic, and this heat keeps the wire glowing for as long as there's ammonia and O2 present.
Chief - Actually, you'd only need ammonium sulfate fertilizer, which is (NH4)2SO4. I did make ammonium nitrate starting with just ammonia. The yield
wasn't anything great, but this was uncontrolled in open air.
|
|
|
| Pages:
1
2 |